Consider the reaction: H2(g) + Br2(g) → 2 HBr(g) The graph shows the concentration of Br2 as a function of time. a. Use the graph to calculate each quantity: (iii) the instantaneous rate of formation of HBr at 50 s
Ch.15 - Chemical Kinetics

Chapter 15, Problem 35b
Consider the reaction: H2( g) + Br2( g) → 2 HBr( g) The graph shows the concentration of Br2 as a function of time.
b. Make a rough sketch of a curve representing the concentration of HBr as a function of time. Assume that the initial concentration of HBr is zero
 Verified step by step guidance
Verified step by step guidance1
Start by understanding that the reaction is a synthesis reaction where hydrogen gas (H_2) and bromine gas (Br_2) react to form hydrogen bromide (HBr).
Since the initial concentration of HBr is zero, the concentration of HBr will increase as the reaction proceeds.
The stoichiometry of the reaction shows that for every mole of Br_2 that reacts, 2 moles of HBr are produced.
As the concentration of Br_2 decreases over time, the concentration of HBr will increase proportionally, but at twice the rate due to the 1:2 stoichiometric ratio.
Sketch a curve that starts at zero and increases over time, showing a steeper slope compared to the decrease in Br_2, reflecting the production of HBr.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Reaction Stoichiometry
Chemical reaction stoichiometry refers to the quantitative relationships between reactants and products in a chemical reaction. In the given reaction, one mole of H<sub>2</sub> reacts with one mole of Br<sub>2</sub> to produce two moles of HBr. Understanding stoichiometry is essential for predicting how the concentrations of reactants and products change over time.
Recommended video:
Guided course

Stoichiometry Concept
Concentration vs. Time Graphs
Concentration vs. time graphs visually represent how the concentration of reactants and products changes during a chemical reaction. For the reaction provided, as Br<sub>2</sub> is consumed, its concentration decreases, while the concentration of HBr increases. Sketching these curves requires understanding the initial conditions and the rates of reaction.
Recommended video:
Guided course

Precipitation: Ksp vs Q Example
Rate of Reaction
The rate of reaction describes how quickly reactants are converted into products over time. It is influenced by factors such as concentration, temperature, and the presence of catalysts. In this reaction, the rate can be inferred from the change in concentration of Br<sub>2</sub> and the corresponding increase in HBr, which is crucial for accurately sketching the concentration curve.
Recommended video:
Guided course
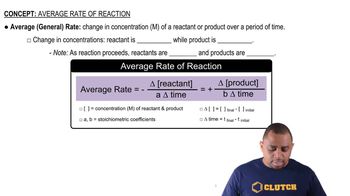
Average Rate of Reaction
Related Practice
Textbook Question
Textbook Question
Consider the reaction: H2(g) + Br2(g) → 2 HBr(g) The graph shows the concentration of Br2 as a function of time.
a. Use the graph to calculate each quantity: (i) the average rate of the reaction between 0 and 25 s
Textbook Question
Consider the reaction: 2 H2O2(aq) → 2 H2O(l ) + O2( g) The graph shows the concentration of H2O2 as a function of time. Use the graph to calculate each quantity: d. If the initial volume of the H2O2 is 1.5 L, what total amount of O2 (in moles) is formed in the first 50 s of reaction?
Textbook Question
This graph shows a plot of the rate of a reaction versus the concentration of the reactant A for the reaction A → products. a. What is the order of the reaction with respect to A?
