Ethanol has a heat of vaporization of 38.56 kJ/mol and a normal boiling point of 78.4 °C. What is the vapor pressure of ethanol at 15 °C?

Methylamine has a vapor pressure of 344 torr at -25 °C and a boiling point of -6.4 °C. What is the ΔHvap for methylamine?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Vapor Pressure
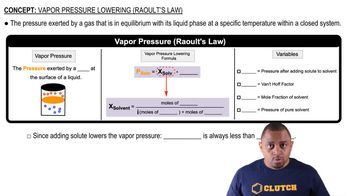
Boiling Point
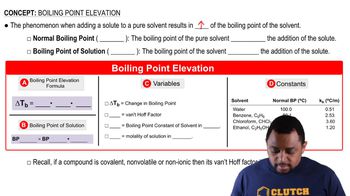
Enthalpy of Vaporization (ΔHvap)
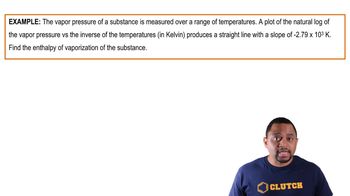
Benzene has a heat of vaporization of 30.72 kJ/mol and a normal boiling point of 80.1 °C. At what temperature does benzene boil when the external pressure is 445 torr?
Carbon disulfide has a vapor pressure of 363 torr at 25 °C and a normal boiling point of 46.3 °C. Find ΔHvap for carbon disulfide.
How much energy is released when 75.2 g of water freezes?
Calculate the amount of heat required to completely sublime 75.0 g of solid dry ice (CO2) at its sublimation temperature. The heat of sublimation for carbon dioxide is 32.3 kJ/mol.
A 10.5-g ice cube at 0°C is placed into 245-g of water. Calculate the temperature change in the water upon the complete melting of the ice. Assume that all of the energy required to melt the ice comes from the water.
