Suppose that 0.95 g of water condenses on a 75.0-g block of iron that is initially at 22 °C. If the heat released during condensation goes only to warming the iron block, what is the final temperature (in °C) of the iron block? (Assume a constant enthalpy of vaporization for water of 44.0 kJ/mol.)
Ch.12 - Liquids, Solids & Intermolecular Forces

Chapter 12, Problem 69
Ethanol has a heat of vaporization of 38.56 kJ/mol and a normal boiling point of 78.4 °C. What is the vapor pressure of ethanol at 15 °C?
 Verified step by step guidance
Verified step by step guidance1
Identify the known values: heat of vaporization (\(\Delta H_{vap}\)) = 38.56 \text{ kJ/mol}, T_1 = 78.4 \degree C = 351.4 \text{ K}, P_1 = 1 \text{ atm}, T_2 = 15 \degree C = 288.15 \text{ K}.
Use the Clausius-Clapeyron equation: \(\ln\left(\frac{P_2}{P_1}\right) = -\frac{\Delta H_{vap}}{R} \left(\frac{1}{T_2} - \frac{1}{T_1}\right)\), where \(R = 8.314 \text{ J/mol K}\).
Convert \(\Delta H_{vap}\) from kJ/mol to J/mol by multiplying by 1000.
Substitute the known values into the Clausius-Clapeyron equation to solve for \(\ln\left(\frac{P_2}{1 \text{ atm}}\right)\).
Exponentiate both sides to solve for \(P_2\), the vapor pressure at 15 \degree C.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Heat of Vaporization
The heat of vaporization is the amount of energy required to convert a unit mass of a liquid into vapor without a change in temperature. For ethanol, this value is 38.56 kJ/mol, indicating the energy needed to overcome intermolecular forces during the phase transition from liquid to gas.
Recommended video:
Guided course
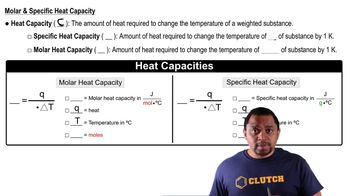
Heat Capacity
Boiling Point
The boiling point of a substance is the temperature at which its vapor pressure equals the external pressure surrounding the liquid. For ethanol, the normal boiling point is 78.4 °C, meaning at this temperature, ethanol will transition from liquid to gas under standard atmospheric pressure.
Recommended video:
Guided course
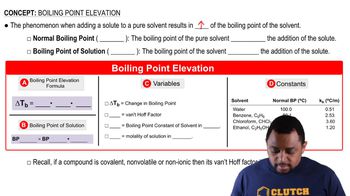
Boiling Point Elevation
Vapor Pressure
Vapor pressure is the pressure exerted by a vapor in equilibrium with its liquid at a given temperature. It reflects the tendency of molecules to escape from the liquid phase into the gas phase. The vapor pressure of ethanol at 15 °C can be estimated using the Clausius-Clapeyron equation, which relates vapor pressure to temperature and heat of vaporization.
Recommended video:
Guided course
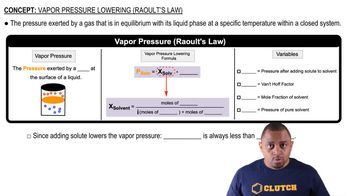
Raoult's Law and Vapor Pressure
Related Practice
Textbook Question
Textbook Question
This table displays the vapor pressure of ammonia at several different temperatures. Use the data to determine the heat of vaporization and normal boiling point of ammonia.
Temperature (K) Pressure (torr)
200 65.3
210 134.3
220 255.7
230 456.0
235 597.0
1
views
Textbook Question
Benzene has a heat of vaporization of 30.72 kJ/mol and a normal boiling point of 80.1 °C. At what temperature does benzene boil when the external pressure is 445 torr?
Textbook Question
Carbon disulfide has a vapor pressure of 363 torr at 25 °C and a normal boiling point of 46.3 °C. Find ΔHvap for carbon disulfide.
1
views
