Textbook Question
How much energy is released when 75.2 g of water freezes?

 Verified step by step guidance
Verified step by step guidance
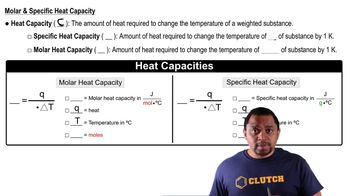


How much energy is released when 75.2 g of water freezes?
Calculate the amount of heat required to completely sublime 75.0 g of solid dry ice (CO2) at its sublimation temperature. The heat of sublimation for carbon dioxide is 32.3 kJ/mol.
How much ice (in grams) would have to melt to absorb 155 kJ of energy?
Consider the phase diagram shown here. Identify the states present at points a through g.
Nitrogen has a normal boiling point of 77.3 K and a melting point (at 1 atm) of 63.1 K. Its critical temperature is 126.2 K and its critical pressure is 2.55×104 torr. It has a triple point at 63.1 K and 94.0 torr. Sketch the phase diagram for nitrogen. Does nitrogen have a stable liquid state at 1 atm?