 Back
BackProblem 78
Silicon has three naturally occurring isotopes (Si-28, Si-29, and Si-30). The mass and natural abundance of Si-28 are 27.9769 amu and 92.2%, respectively. The mass and natural abundance of Si-29 are 28.9765 amu and 4.67%, respectively. Find the mass and natural abundance of Si-30.
Problem 79
Use the mass spectrum of europium to determine the atomic mass of europium.
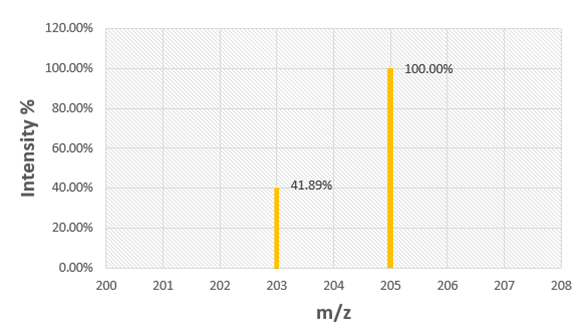
Problem 80
Use the mass spectrum of rubidium to determine the atomic mass of rubidium.
Problem 81
How many sulfur atoms are there in 5.52 mol of sulfur?
Problem 82
How many moles of aluminum do 3.7×1024 aluminum atoms represent?
Problem 83
What is the amount, in moles, of each elemental sample? a. 11.8 g Ar b. 3.55 g Zn c. 26.1 g Ta d. 0.211 g Li
Problem 84a,b,d
What is the mass, in grams, of each elemental sample? a. 2.3×10–3 mol Sb b. 0.0355 mol Ba d. 1.3 mol W
Problem 84c
What is the mass, in grams, of each elemental sample? c. 43.9 mol Xe
Problem 85
How many silver atoms are there in 3.78 g of silver?
Problem 86
What is the mass of 4.91×1021 platinum atoms?
Problem 87
Calculate the number of atoms in each sample. a. 5.18 g P
Problem 88
Calculate the number of atoms in each sample. a. 14.955 g Cr
Problem 89a,b
Calculate the mass, in grams, of each sample. a. 1.1×1023 gold atoms b. 2.82×1022 helium atoms
Problem 89c
Calculate the mass, in grams, of each sample. c. 1.8×1023 lead atoms
Problem 89d
Calculate the mass, in grams, of each sample. d. 7.9×1021 uranium atoms
Problem 90
Calculate the mass, in kg, of each sample. a. 7.55×1026 cadmium atoms b. 8.15×1027 nickel atoms c. 1.22×1027 manganese atoms d. 5.48×1029 lithium atoms
Problem 91
How many carbon atoms are there in a diamond (pure carbon) with a mass of 52 mg?
Problem 92
How many helium atoms are there in a helium blimp containing 536 kg of helium?
Problem 93
Calculate the average mass, in grams, of one platinum atom.
Problem 94
Using scanning tunneling microscopy, scientists at IBM wrote the initials of their company with 35 individual xenon atoms (as shown below). Calculate the total mass of these letters in grams.
Problem 95
A 7.83 g sample of HCN contains 0.290 g of H and 4.06 g of N. Find the mass of carbon in a sample of HCN with a mass of 3.37 g.
Problem 96
The ratio of sulfur to oxygen by mass in SO2 is 1.0:1.0. a. Find the ratio of sulfur to oxygen by mass in SO3. b. Find the ratio of sulfur to oxygen by mass in S2O.
Problem 97
The ratio of oxygen to carbon by mass in carbon monoxide is 1.33:1.00. Find the formula of an oxide of carbon in which the ratio by mass of oxygen to carbon is 2.00:1.00.
Problem 98
The ratio of the mass of a nitrogen atom to the mass of an atom of 12C is 7:6, and the ratio of the mass of nitrogen to oxygen in N2O is 7:4. Find the mass of 1 mol of oxygen atoms.
Problem 99
An a particle, 4He2 + , has a mass of 4.00151 amu. Find the value of its charge-to-mass ratio in C/kg.
Problem 100
Naturally occurring iodine has an atomic mass of 126.9045 amu. A 12.3849 g sample of iodine is accidentally contaminated with an additional 1.00070 g of 129I, a synthetic radioisotope of iodine used in the treatment of certain diseases of the thyroid gland. The mass of 129I is 128.9050 amu. Find the apparent 'atomic mass' of the contaminated iodine.
Problem 101
Use the mass spectrum of lead to estimate the atomic mass of lead. Estimate the mass and percent intensity values from the graph to three significant figures.

Problem 102
Use the mass spectrum of mercury to estimate the atomic mass of mercury. Estimate the masses and percent intensity values from the graph to three significant figures.
Problem 103
Nuclei with the same number of neutrons but different mass numbers are called isotones. Write the symbols of four isotones of 236Th.
Problem 104
Fill in the blanks to complete the table. Symbol Z A Number of p_x001E_ Number of e_x001D_ Number of n Charge Si 14 ____ ____ 14 14 ____ S2- ____ 32 ____ ____ ____ 2- Cu2+ ____ ____ ____ ____ 34 2+ ____ 15 ____ ____ 15 16 ____
