Textbook Question
Calculate the mass, in grams, of each sample. a. 1.1×1023 gold atoms b. 2.82×1022 helium atoms

 Verified step by step guidance
Verified step by step guidance

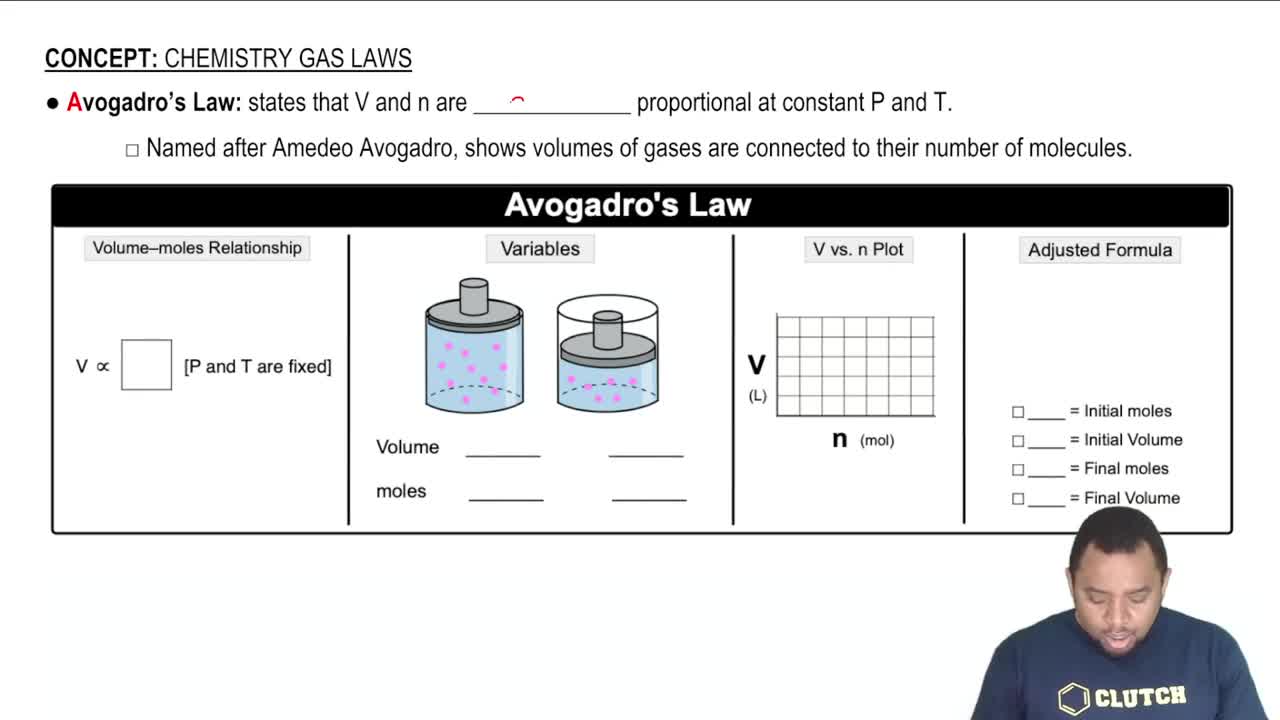

Calculate the mass, in grams, of each sample. a. 1.1×1023 gold atoms b. 2.82×1022 helium atoms
Calculate the mass, in grams, of each sample. c. 1.8×1023 lead atoms
Calculate the mass, in grams, of each sample. d. 7.9×1021 uranium atoms
How many carbon atoms are there in a diamond (pure carbon) with a mass of 52 mg?
How many helium atoms are there in a helium blimp containing 536 kg of helium?
Calculate the average mass, in grams, of one platinum atom.