Textbook Question
Predict a likely mode of decay for each unstable nuclide. a. Mo-109
1
views

 Verified step by step guidance
Verified step by step guidance
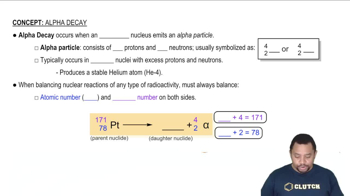


Predict a likely mode of decay for each unstable nuclide. a. Mo-109
Predict a likely mode of decay for each unstable nuclide. b. Ru-90 c. P-27 d. Sn-100
Predict a likely mode of decay for each unstable nuclide. a. Sb-132 b. Te-139 d. Ba-123
Which nuclide in each pair would you expect to have the longer half-life? a. Cs-113 or Cs-125 b. Fe-62 or Fe-70
Which nuclide in each pair would you expect to have the longer half-life? a. Cs-149 or Cs-139 b. Fe-45 or Fe-52
One of the nuclides in spent nuclear fuel is U-235, an alpha emitter with a half-life of 703 million years. How long will it take for the amount of U-235 to reach 10.0% of its initial amount?