Textbook Question
Write the nuclear equation for the most likely mode of decay for each unstable nuclide. a. Ru-114 c. Zn-58 d. Ne-31

 Verified step by step guidance
Verified step by step guidance

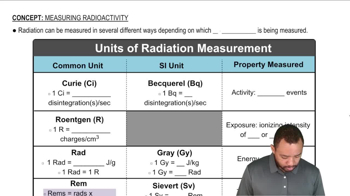
Write the nuclear equation for the most likely mode of decay for each unstable nuclide. a. Ru-114 c. Zn-58 d. Ne-31
Write the nuclear equation for the most likely mode of decay for each unstable nuclide. b. Ra-216
Write the nuclear equation for the most likely mode of decay for each unstable nuclide. a. Kr-74 b. Th-221 c. Ar-44 d. Nb-85