Complete each nuclear equation and calculate the energy change (in J/mol of reactant) associated with each (Be-9 = 9.012182 amu, Bi-209 = 208.980384 amu, He-4 = 4.002603 amu, Li-6 = 6.015122 amu, Ni-64 = 63.927969 amu, Rg-272 = 272.1535 amu, Ta-179 = 178.94593 amu, and W-179 = 178.94707 amu). a. _____ + 94Be → 63Li + 42He
Ch.20 - Radioactivity and Nuclear Chemistry

Chapter 20, Problem 79a,c,d
Write the nuclear equation for the most likely mode of decay for each unstable nuclide. a. Ru-114 c. Zn-58 d. Ne-31
 Verified step by step guidance
Verified step by step guidance1
Identify the type of decay: Determine the most likely mode of decay for Ru-114. Since it is a neutron-rich nuclide, beta decay (β-) is a common mode of decay for such isotopes.
Write the beta decay equation: In beta decay, a neutron is converted into a proton, and a beta particle (electron) and an antineutrino are emitted. The atomic number increases by 1, while the mass number remains the same.
Determine the daughter nuclide: For Ru-114, the atomic number of ruthenium (Ru) is 44. After beta decay, the atomic number becomes 45, which corresponds to the element rhodium (Rh).
Write the nuclear equation: Represent the decay process as follows: \[ \text{^{114}_{44}Ru} \rightarrow \text{^{114}_{45}Rh} + \beta^- + \bar{\nu}_e \]
Verify the equation: Ensure that the mass numbers and atomic numbers are balanced on both sides of the equation. The mass number is 114 on both sides, and the atomic numbers are 44 (Ru) and 45 (Rh) + (-1 for the beta particle).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclear Decay
Nuclear decay is the process by which an unstable atomic nucleus loses energy by emitting radiation. This can occur through various modes, including alpha decay, beta decay, and gamma decay. Each mode involves the transformation of the nucleus, resulting in the emission of particles or electromagnetic radiation, leading to the formation of a different nuclide.
Recommended video:
Guided course
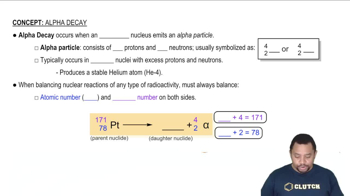
Alpha Decay
Nuclear Equations
Nuclear equations represent the changes that occur during nuclear reactions, including decay processes. They typically show the initial nuclide, the emitted particles, and the resulting nuclide. For example, in beta decay, a neutron is converted into a proton, and an electron (beta particle) is emitted, which can be represented in a balanced equation format.
Recommended video:
Guided course
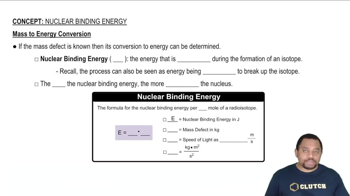
Nuclear Binding Energy
Isotopes and Stability
Isotopes are variants of a chemical element that have the same number of protons but different numbers of neutrons. The stability of an isotope depends on the ratio of neutrons to protons; an unstable isotope, like Ru-114, may undergo decay to achieve a more stable configuration. Understanding the stability of isotopes is crucial for predicting their decay modes and writing accurate nuclear equations.
Recommended video:
Guided course
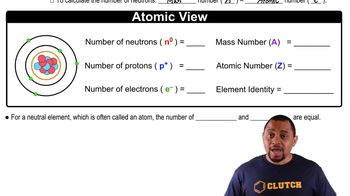
Isotopes
Related Practice
Textbook Question
Textbook Question
Complete each nuclear equation and calculate the energy change (in J/mol of reactant) associated with each (Al-27 = 26.981538 amu, Am-241 = 241.056822 amu, He-4 = 4.002603 amu, Np-237 = 237.048166 amu, P-30 = 29.981801 amu, S-32 = 31.972071 amu, and Si-29 = 28.976495 amu).
a. 2713Al + 42He → 3015P + ____
Textbook Question
Write the nuclear equation for the most likely mode of decay for each unstable nuclide. b. Ra-216
Textbook Question
Write the nuclear equation for the most likely mode of decay for each unstable nuclide. a. Kr-74 b. Th-221 c. Ar-44 d. Nb-85
