Calculate ΔSsurr at the indicated temperature for each reaction. d. ΔH°rxn = +114 kJ; 77 K
Ch.18 - Free Energy and Thermodynamics

Chapter 18, Problem 41c
Given the values of ΔH°rxn, ΔS°rxn, and T, determine ΔSuniv and predict whether or not each reaction is spontaneous. (Assume that all reactants and products are in their standard states.) c. ΔH°rxn = -115 kJ; ΔS°rxn = -263 J/K; T = 298 K
 Verified step by step guidance
Verified step by step guidance1
Calculate the change in entropy of the universe (ΔSuniv) using the formula: ΔSuniv = ΔSrxn + ΔSsurr. Here, ΔSsurr is the change in entropy of the surroundings.
Convert ΔHrxn from kilojoules to joules to ensure consistency in units for the calculations. Recall that 1 kJ = 1000 J.
Calculate ΔSsurr using the formula: ΔSsurr = -ΔHrxn / T. This formula arises from the definition of entropy change in the surroundings due to heat transfer at constant temperature.
Substitute the values of ΔHrxn (converted to joules), ΔSrxn, and T into the formula to find ΔSuniv.
Determine the spontaneity of the reaction by checking the sign of ΔSuniv. If ΔSuniv is positive, the reaction is spontaneous; if it is negative, the reaction is non-spontaneous.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Gibbs Free Energy
Gibbs Free Energy (G) is a thermodynamic potential that helps predict the spontaneity of a reaction at constant temperature and pressure. It is calculated using the equation ΔG = ΔH - TΔS, where ΔH is the change in enthalpy, T is the temperature in Kelvin, and ΔS is the change in entropy. A negative ΔG indicates that a reaction is spontaneous, while a positive ΔG suggests it is non-spontaneous.
Recommended video:
Guided course
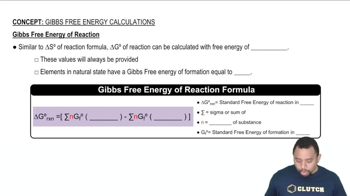
Gibbs Free Energy of Reactions
Entropy (ΔS)
Entropy (ΔS) is a measure of the disorder or randomness in a system. In the context of chemical reactions, it quantifies the change in disorder as reactants transform into products. A positive ΔS indicates an increase in disorder, which generally favors spontaneity, while a negative ΔS suggests a decrease in disorder, which can hinder spontaneity.
Recommended video:
Guided course
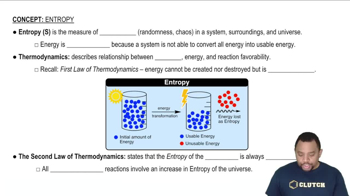
Entropy in Thermodynamics
Enthalpy (ΔH)
Enthalpy (ΔH) is a measure of the total heat content of a system and reflects the energy changes during a chemical reaction. A negative ΔH (exothermic reaction) indicates that the reaction releases heat, which can favor spontaneity. Conversely, a positive ΔH (endothermic reaction) absorbs heat, potentially making the reaction non-spontaneous unless compensated by a sufficiently large positive ΔS.
Recommended video:
Guided course

Enthalpy of Formation
Related Practice
Textbook Question
Textbook Question
Given the values of ΔH°rxn, ΔS°rxn, and T, determine ΔSuniv and predict whether or not each reaction is spontaneous. (Assume that all reactants and products are in their standard states.) a. ΔH°rxn = +115 kJ; ΔS°rxn = -263 J/K; T = 298 K
Textbook Question
Given the values of ΔH°rxn, ΔS°rxn, and T, determine ΔSuniv and predict whether or not each reaction is spontaneous. (Assume that all reactants and products are in their standard states.) a. ΔH°rxn = -95 kJ; ΔS°rxn = -157 J/K; T = 298 K
Textbook Question
Given the values of ΔH°rxn, ΔS°rxn, and T, determine ΔSuniv and predict whether or not each reaction is spontaneous. (Assume that all reactants and products are in their standard states.) c. ΔH°rxn = +95 kJ; ΔS°rxn = -157 J/K; T = 298 K
