Without doing any calculations, determine the signs of ΔSsys and ΔSsurr for each chemical reaction. In addition, predict under what temperatures (all temperatures, low temperatures, or high temperatures), if any, the reaction is spontaneous. c. 2 N2(g) + O2(g) → 2 N2O(g) ΔH°rxn = +163.2 kJ
Ch.18 - Free Energy and Thermodynamics

Chapter 18, Problem 39d
Calculate ΔSsurr at the indicated temperature for each reaction. d. ΔH°rxn = +114 kJ; 77 K
 Verified step by step guidance
Verified step by step guidance1
Identify the formula to calculate the change in entropy of the surroundings, which is given by \( \Delta S_{\text{surr}} = -\frac{\Delta H_{\text{rxn}}}{T} \).
Note that \( \Delta H_{\text{rxn}} \) is given as +114 kJ. Since entropy is typically expressed in J/K, convert \( \Delta H_{\text{rxn}} \) from kJ to J by multiplying by 1000.
The temperature \( T \) is given as 77 K. Ensure that the temperature is in Kelvin, which it is in this case.
Substitute the values into the formula: \( \Delta S_{\text{surr}} = -\frac{114,000 \text{ J}}{77 \text{ K}} \).
Simplify the expression to find \( \Delta S_{\text{surr}} \).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Entropy (ΔS)
Entropy is a measure of the disorder or randomness in a system. In thermodynamics, it quantifies the amount of energy in a physical system that is not available to do work. A positive change in entropy (ΔS > 0) indicates an increase in disorder, while a negative change (ΔS < 0) suggests a decrease in disorder. Understanding entropy is crucial for predicting the spontaneity of reactions.
Recommended video:
Guided course
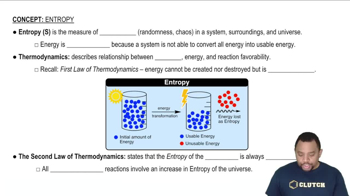
Entropy in Thermodynamics
Enthalpy (ΔH)
Enthalpy is a thermodynamic property that reflects the total heat content of a system. It is defined as the internal energy plus the product of pressure and volume (H = U + PV). The change in enthalpy (ΔH) during a reaction indicates whether the reaction is exothermic (ΔH < 0) or endothermic (ΔH > 0). This concept is essential for calculating the surrounding entropy (ΔS surr) using the relationship between enthalpy and temperature.
Recommended video:
Guided course

Enthalpy of Formation
Gibbs Free Energy and Surrounding Entropy
The Gibbs free energy (G) combines enthalpy and entropy to determine the spontaneity of a reaction at constant temperature and pressure. The relationship ΔG = ΔH - TΔS allows us to calculate the change in entropy of the surroundings (ΔS surr) using the enthalpy change and the temperature. A reaction is spontaneous if ΔG is negative, which is influenced by both ΔH and ΔS surr.
Recommended video:
Guided course
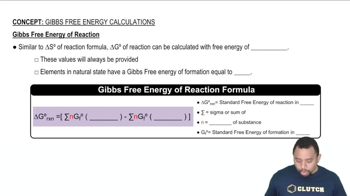
Gibbs Free Energy of Reactions
Related Practice
Textbook Question
Textbook Question
Given the values of ΔH°rxn, ΔS°rxn, and T, determine ΔSuniv and predict whether or not each reaction is spontaneous. (Assume that all reactants and products are in their standard states.) a. ΔH°rxn = +115 kJ; ΔS°rxn = -263 J/K; T = 298 K
Textbook Question
Given the values of ΔH°rxn, ΔS°rxn, and T, determine ΔSuniv and predict whether or not each reaction is spontaneous. (Assume that all reactants and products are in their standard states.) c. ΔH°rxn = -115 kJ; ΔS°rxn = -263 J/K; T = 298 K
