Given the values of ΔH°rxn, ΔS°rxn, and T, determine ΔSuniv and predict whether or not each reaction is spontaneous. (Assume that all reactants and products are in their standard states.) a. ΔH°rxn = +115 kJ; ΔS°rxn = -263 J/K; T = 298 K
Ch.18 - Free Energy and Thermodynamics

Chapter 18, Problem 42
Given the values of ΔH°rxn, ΔS°rxn, and T, determine ΔS°univ and predict whether or not each reaction is spontaneous. (Assume that all reactants and products are in their standard states.) a. ΔH°rxn = -95 kJ; ΔS°rxn = -157 J/K; T = 855 K b. ΔH°rxn = -95 kJ; ΔS°rxn = +157 J/K; T = 398 K
 Verified step by step guidance
Verified step by step guidance1
Convert \( \Delta H^\circ_{rxn} \) from kJ to J by multiplying by 1000, since \( \Delta S^\circ_{rxn} \) is given in J/K.
Calculate \( \Delta S^\circ_{univ} \) using the formula: \( \Delta S^\circ_{univ} = \Delta S^\circ_{rxn} - \frac{\Delta H^\circ_{rxn}}{T} \).
Substitute the given values for \( \Delta H^\circ_{rxn} \), \( \Delta S^\circ_{rxn} \), and \( T \) into the formula for each reaction.
Determine the sign of \( \Delta S^\circ_{univ} \) for each reaction. If \( \Delta S^\circ_{univ} > 0 \), the reaction is spontaneous; if \( \Delta S^\circ_{univ} < 0 \), the reaction is non-spontaneous.
Interpret the results to predict the spontaneity of each reaction based on the calculated \( \Delta S^\circ_{univ} \).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
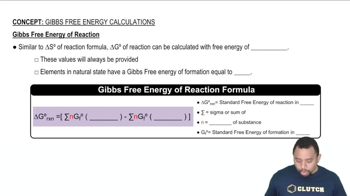
Gibbs Free Energy
Gibbs Free Energy (G) is a thermodynamic potential that helps predict the spontaneity of a reaction at constant temperature and pressure. It is defined as G = ΔH - TΔS, where ΔH is the change in enthalpy, T is the temperature in Kelvin, and ΔS is the change in entropy. A reaction is spontaneous if ΔG is negative, indicating that the process can occur without external energy input.
Recommended video:
Guided course
Gibbs Free Energy of Reactions
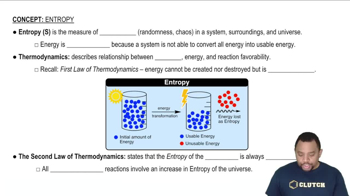
Entropy (ΔS)
Entropy (ΔS) is a measure of the disorder or randomness in a system. A positive ΔS indicates an increase in disorder, while a negative ΔS suggests a decrease in disorder. In the context of reactions, changes in entropy can significantly influence the spontaneity of a reaction, especially when combined with temperature in the Gibbs Free Energy equation.
Recommended video:
Guided course
Entropy in Thermodynamics
Enthalpy (ΔH)
Enthalpy (ΔH) is the heat content of a system at constant pressure, reflecting the energy absorbed or released during a chemical reaction. A negative ΔH indicates an exothermic reaction, where heat is released, while a positive ΔH indicates an endothermic reaction, where heat is absorbed. The sign and magnitude of ΔH are crucial for determining the overall energy change and spontaneity of a reaction when analyzed alongside ΔS and temperature.
Recommended video:
Guided course

Enthalpy of Formation
Related Practice
Textbook Question
Textbook Question
Given the values of ΔH°rxn, ΔS°rxn, and T, determine ΔSuniv and predict whether or not each reaction is spontaneous. (Assume that all reactants and products are in their standard states.) c. ΔH°rxn = -115 kJ; ΔS°rxn = -263 J/K; T = 298 K
Textbook Question
Given the values of ΔH°rxn, ΔS°rxn, and T, determine ΔSuniv and predict whether or not each reaction is spontaneous. (Assume that all reactants and products are in their standard states.) a. ΔH°rxn = -95 kJ; ΔS°rxn = -157 J/K; T = 298 K
Textbook Question
Given the values of ΔH°rxn, ΔS°rxn, and T, determine ΔSuniv and predict whether or not each reaction is spontaneous. (Assume that all reactants and products are in their standard states.) c. ΔH°rxn = +95 kJ; ΔS°rxn = -157 J/K; T = 298 K
