Without doing any calculations, determine the signs of ΔSsys and ΔSsurr for each chemical reaction. In addition, predict under what temperatures (all temperatures, low temperatures, or high temperatures), if any, the reaction is spontaneous. c. 2 N2(g) + O2(g) → 2 N2O(g) ΔH°rxn = +163.2 kJ
Ch.18 - Free Energy and Thermodynamics

Chapter 18, Problem 41
Given the values of ΔH° rxn, ΔS° rxn, and T, determine ΔSuniv and predict whether each reaction is spontaneous. Assume that all reactants and products are in their standard states. b. ΔH° rxn = -115 kJ; ΔS° rxn = +263 J/K; T = 298 K; d. ΔH° rxn = -115 kJ; ΔS° rxn = -263 J/K; T = 615 K.
 Verified step by step guidance
Verified step by step guidance1
Convert \( \Delta H^\circ_{rxn} \) from kJ to J by multiplying by 1000, since \( \Delta S^\circ_{rxn} \) is given in J/K.
Calculate \( \Delta G^\circ_{rxn} \) using the formula \( \Delta G^\circ_{rxn} = \Delta H^\circ_{rxn} - T \Delta S^\circ_{rxn} \).
Determine \( \Delta S_{univ} \) using the relationship \( \Delta S_{univ} = -\frac{\Delta G^\circ_{rxn}}{T} \).
Evaluate the sign of \( \Delta S_{univ} \): if positive, the reaction is spontaneous; if negative, it is non-spontaneous.
Repeat the above steps for each set of given values to determine spontaneity for each reaction.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Gibbs Free Energy
Gibbs Free Energy (G) is a thermodynamic potential that helps predict the spontaneity of a reaction at constant temperature and pressure. It is defined as G = ΔH - TΔS, where ΔH is the change in enthalpy, T is the temperature in Kelvin, and ΔS is the change in entropy. A reaction is spontaneous if ΔG is negative, indicating that the process can occur without external energy input.
Recommended video:
Guided course
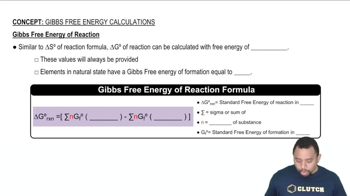
Gibbs Free Energy of Reactions
Entropy (ΔS)
Entropy (ΔS) is a measure of the disorder or randomness in a system. A positive ΔS indicates an increase in disorder, which generally favors spontaneity, while a negative ΔS suggests a decrease in disorder. In the context of reactions, the change in entropy can significantly influence the overall spontaneity when combined with temperature in the Gibbs Free Energy equation.
Recommended video:
Guided course
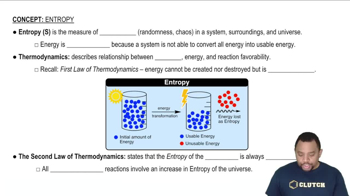
Entropy in Thermodynamics
Enthalpy (ΔH)
Enthalpy (ΔH) is the total heat content of a system and reflects the energy change during a chemical reaction. A negative ΔH indicates an exothermic reaction, releasing heat and often favoring spontaneity, while a positive ΔH indicates an endothermic reaction, absorbing heat. The interplay between ΔH and ΔS at a given temperature is crucial for determining the spontaneity of a reaction.
Recommended video:
Guided course

Enthalpy of Formation
Related Practice
Textbook Question
Textbook Question
Calculate ΔSsurr at the indicated temperature for each reaction. d. ΔH°rxn = +114 kJ; 77 K
Textbook Question
Given the values of ΔH°rxn, ΔS°rxn, and T, determine ΔSuniv and predict whether or not each reaction is spontaneous. (Assume that all reactants and products are in their standard states.) a. ΔH°rxn = +115 kJ; ΔS°rxn = -263 J/K; T = 298 K
Textbook Question
Given the values of ΔH°rxn, ΔS°rxn, and T, determine ΔSuniv and predict whether or not each reaction is spontaneous. (Assume that all reactants and products are in their standard states.) c. ΔH°rxn = -115 kJ; ΔS°rxn = -263 J/K; T = 298 K
