Determine whether or not the mixing of each pair of solutions results in a buffer. e. 125.0 mL of 0.15 M NH3; 150.0 mL of 0.20 M NaOH

Determine whether or not the mixing of each pair of solutions results in a buffer. e. 105.0 mL of 0.15 M CH3NH2; 95.0 mL of 0.10 M HCl
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Buffer Solutions

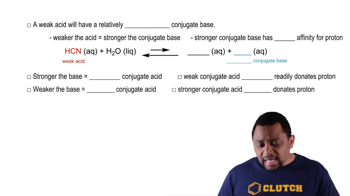
Weak Bases and Their Conjugate Acids
Stoichiometry of Acid-Base Reactions

Determine whether or not the mixing of each pair of solutions results in a buffer. a. 75.0 mL of 0.10 M HF; 55.0 mL of 0.15 M NaF b. 150.0 mL of 0.10 M HF; 135.0 mL of 0.175 M HCl d. 125.0 mL of 0.15 M CH3NH2; 120.0 mL of 0.25 M CH3NH3Cl
Determine whether or not the mixing of each pair of solutions results in a buffer. c. 165.0 mL of 0.10 M HF; 135.0 mL of 0.050 M KOH
Blood is buffered by carbonic acid and the bicarbonate ion. Normal blood plasma is 0.024 M in HCO3- and 0.0012 M H2CO3 (pKa1 for H2CO3 at body temperature is 6.1).
a. What is the pH of blood plasma?
Blood is buffered by carbonic acid and the bicarbonate ion. Normal blood plasma is 0.024 M in HCO3- and 0.0012 M H2CO3 (pKa1 for H2CO3 at body temperature is 6.1).
c. Given the volume from part (b), what mass of NaOH can be neutralized before the pH rises above 7.8?
