Determine whether or not the mixing of each pair of solutions results in a buffer. c. 50.0 mL of 0.15 M HF; 20.0 mL of 0.15 M NaOH
Ch.17 - Aqueous Ionic Equilibrium

Chapter 17, Problem 54a,b,d
Determine whether or not the mixing of each pair of solutions results in a buffer. a. 75.0 mL of 0.10 M HF; 55.0 mL of 0.15 M NaF b. 150.0 mL of 0.10 M HF; 135.0 mL of 0.175 M HCl d. 125.0 mL of 0.15 M CH3NH2; 120.0 mL of 0.25 M CH3NH3Cl
 Verified step by step guidance
Verified step by step guidance1
Identify the components needed for a buffer solution: a weak acid and its conjugate base, or a weak base and its conjugate acid.
Recognize that HF is a weak acid, which is a potential component of a buffer solution.
Note that HCl is a strong acid, which will completely dissociate in solution and does not form a buffer with HF.
Calculate the moles of HF: \( \text{moles of HF} = 0.150 \text{ L} \times 0.10 \text{ M} \).
Calculate the moles of HCl: \( \text{moles of HCl} = 0.135 \text{ L} \times 0.175 \text{ M} \).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Buffer Solutions
A buffer solution is a system that resists changes in pH upon the addition of small amounts of acid or base. It typically consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Buffers are crucial in maintaining stable pH levels in various chemical and biological processes.
Recommended video:
Guided course
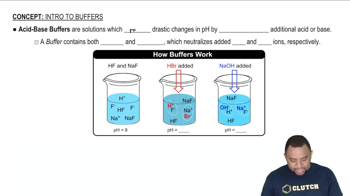
Buffer Solutions
Weak Acids and Strong Acids
Weak acids, like HF (hydrofluoric acid), partially dissociate in solution, establishing an equilibrium between the undissociated acid and its ions. In contrast, strong acids, such as HCl (hydrochloric acid), completely dissociate in solution. The presence of a strong acid can disrupt the equilibrium of a weak acid, affecting the buffer capacity.
Recommended video:
Guided course
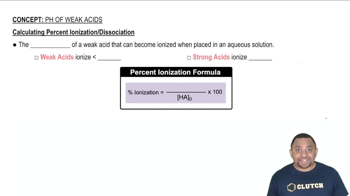
Calculating Percent Ionization of Weak Acids
pH and Acid-Base Reactions
pH is a measure of the hydrogen ion concentration in a solution, indicating its acidity or basicity. Acid-base reactions involve the transfer of protons (H+) between species, influencing the pH. Understanding how the mixing of different acids affects the overall pH is essential for determining whether a buffer is formed.
Recommended video:
Guided course
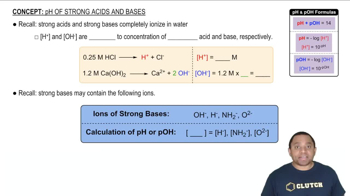
pH of Strong Acids and Bases
Related Practice
Textbook Question
1
views
Textbook Question
Determine whether or not the mixing of each pair of solutions results in a buffer. d. 175.0 mL of 0.10 M NH3; 150.0 mL of 0.12 M NaOH
1
views
Textbook Question
Determine whether or not the mixing of each pair of solutions results in a buffer. e. 125.0 mL of 0.15 M NH3; 150.0 mL of 0.20 M NaOH
1
views
Textbook Question
Determine whether or not the mixing of each pair of solutions results in a buffer. c. 165.0 mL of 0.10 M HF; 135.0 mL of 0.050 M KOH
1
views
Textbook Question
Determine whether or not the mixing of each pair of solutions results in a buffer. e. 105.0 mL of 0.15 M CH3NH2; 95.0 mL of 0.10 M HCl
1
views
Textbook Question
Blood is buffered by carbonic acid and the bicarbonate ion. Normal blood plasma is 0.024 M in HCO3- and 0.0012 M H2CO3 (pKa1 for H2CO3 at body temperature is 6.1).
a. What is the pH of blood plasma?
