This reaction was monitored as a function of time: AB → A + B A plot of 1/[AB] versus time yields a straight line with a slope of +0.55/Ms.
a. What is the value of the rate constant (k) for this reaction at this temperature?

 Verified step by step guidance
Verified step by step guidance

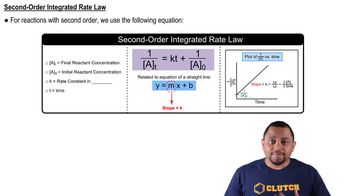

This reaction was monitored as a function of time: AB → A + B A plot of 1/[AB] versus time yields a straight line with a slope of +0.55/Ms.
a. What is the value of the rate constant (k) for this reaction at this temperature?
This reaction was monitored as a function of time: AB → A + B A plot of 1/[AB] versus time yields a straight line with a slope of +0.55/Ms. b. Write the rate law for the reaction.
This reaction was monitored as a function of time: AB → A + B A plot of 1/[AB] versus time yields a straight line with a slope of +0.55/Ms. c. What is the half-life when the initial concentration is 0.55 M?
The decomposition of XY is second order in XY and has a rate constant of 7.02⨉10-3 M-1• s-1 at a certain temperature. a. What is the half-life for this reaction at an initial concentration of 0.100 M?