Textbook Question
An isotonic solution contains 0.90% NaCl mass to volume. Calculate the percent mass to volume for isotonic solutions containing each solute at 25 °C. Assume a van't Hoff factor of 1.9 for all ionic solutes. a. KCl

 Verified step by step guidance
Verified step by step guidance
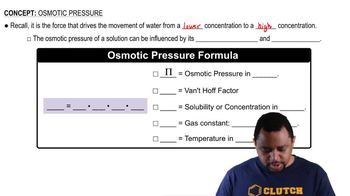
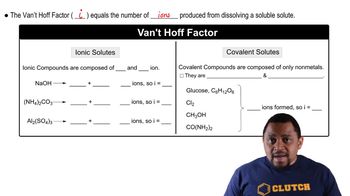
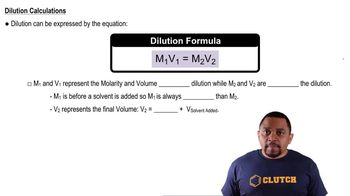
An isotonic solution contains 0.90% NaCl mass to volume. Calculate the percent mass to volume for isotonic solutions containing each solute at 25 °C. Assume a van't Hoff factor of 1.9 for all ionic solutes. a. KCl
When HNO2 is dissolved in water, it partially dissociates according to the equation HNO2 ⇌ H+ + NO2-. A solution is prepared that contains 7.050 g of HNO2 in 1.000 kg of water. Its freezing point is -0.2929 °C. Calculate the fraction of HNO2 that has dissociated.
A solution of a nonvolatile solute in water has a boiling point of 375.3 K. Calculate the vapor pressure of water above this solution at 338 K. The vapor pressure of pure water at this temperature is 0.2467 atm.