Determine whether each pair of compounds forms a homogeneous solution when combined. For those that form homogeneous solutions, indicate the type of forces that are involved. d. CH3CH2OH and H2O
Ch.11 - Liquids, Solids & Intermolecular Forces

Chapter 11, Problem 48
Why does water wet some surfaces and bead up on others, while mercury beads up on almost all surfaces?
 Verified step by step guidance
Verified step by step guidance1
Understand the concept of surface tension and how it relates to the cohesive and adhesive forces between molecules.
Recognize that water has strong hydrogen bonds, which contribute to its high surface tension, allowing it to wet surfaces where adhesive forces are stronger than cohesive forces.
Identify that mercury has metallic bonds, which are much stronger than the adhesive forces with most surfaces, leading to high cohesion and causing it to bead up.
Consider the role of surface energy: surfaces with high surface energy (like glass) are more likely to be wetted by water, while low surface energy surfaces (like wax) cause water to bead up.
Apply the concept of contact angle: a low contact angle indicates wetting (water spreads out), while a high contact angle indicates beading (water forms droplets).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Surface Tension
Surface tension is a physical property of liquids that describes the elastic-like force at the surface of a liquid. It arises from the cohesive forces between liquid molecules, which are stronger at the surface due to the lack of neighboring molecules above. This phenomenon explains why water forms droplets on certain surfaces and why it can 'wet' others, depending on the balance between cohesive and adhesive forces.
Recommended video:
Guided course
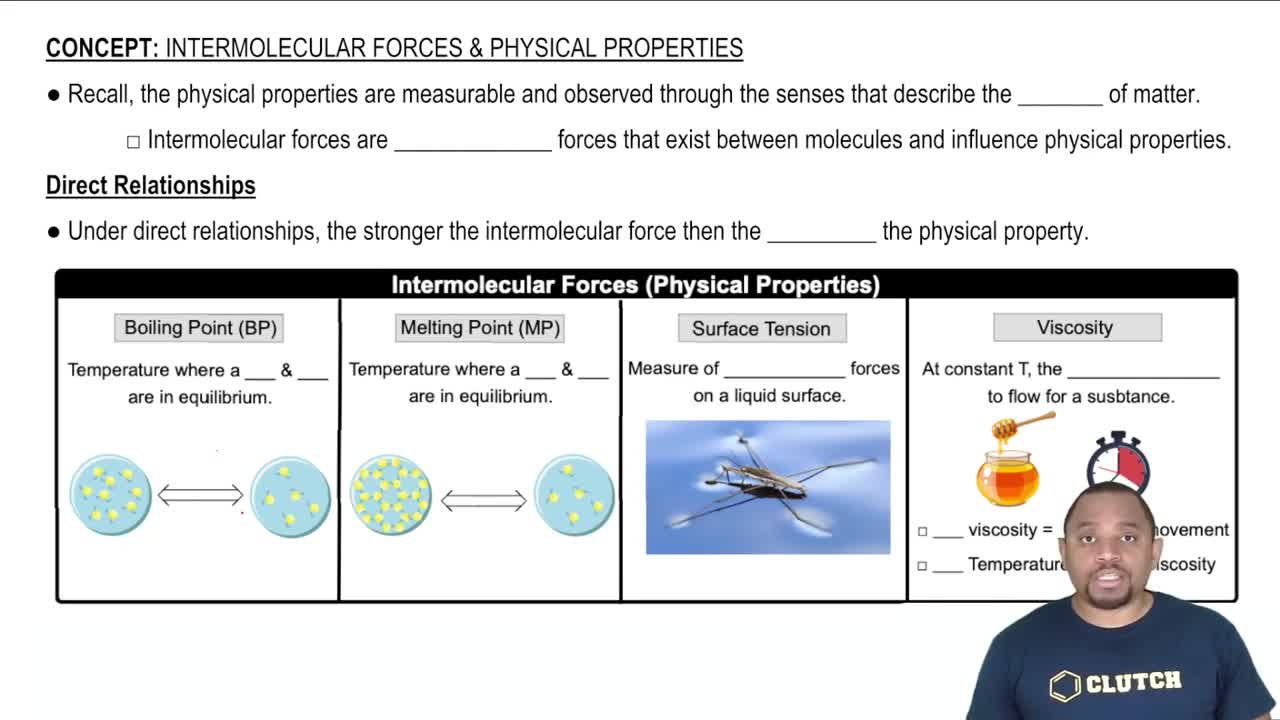
Intermolecular Forces and Properties
Adhesion and Cohesion
Adhesion refers to the attraction between different substances, while cohesion is the attraction between like molecules. In the case of water, if the adhesive forces between water molecules and a surface are stronger than the cohesive forces among the water molecules, the water will spread out and wet the surface. Conversely, if cohesion dominates, water will bead up, as seen on hydrophobic surfaces.
Recommended video:
Guided course

Intermolecular Forces and Properties
Hydrophobic and Hydrophilic Surfaces
Hydrophobic surfaces repel water and do not allow it to spread, leading to beading, while hydrophilic surfaces attract water, promoting spreading and wetting. The chemical composition and structure of a surface determine its hydrophobicity or hydrophilicity. Mercury, being a dense liquid with strong cohesive forces, beads up on most surfaces due to its high surface tension and lack of adhesive interaction with them.
Recommended video:
Guided course

Photoelectric Effect
Related Practice
Textbook Question
Textbook Question
Determine whether each pair of compounds forms a homogeneous solution when combined. For those that form homogeneous solutions, indicate the type of forces that are involved. a. CH3CH2CH2CH2CH3 and CH3CH2CH2CH2CH2CH3 b. CBr4 and H2O c. LiNO3 and H2O d. CH3OH and CH3CH2CH2CH2CH3
Textbook Question
Which compound would you expect to have greater surface tension: acetone [(CH3)2CO] or water (H2O)? Explain.
Textbook Question
The structures of two isomers of heptane are shown. Which of these two compounds would you expect to have the greater viscosity?
Textbook Question
Explain why the viscosity of multigrade motor oils is less temperature-dependent than that of single-grade motor oils.
