In each pair of compounds, pick the one with the higher vapor pressure at a given temperature. Explain your reasoning. b. CH3CH2CH2OH or CH3OH
Ch.11 - Liquids, Solids & Intermolecular Forces

Chapter 11, Problem 46
Determine whether each pair of compounds forms a homogeneous solution when combined. For those that form homogeneous solutions, indicate the type of forces that are involved. a. CH3CH2CH2CH2CH3 and CH3CH2CH2CH2CH2CH3 b. CBr4 and H2O c. LiNO3 and H2O d. CH3OH and CH3CH2CH2CH2CH3
 Verified step by step guidance
Verified step by step guidance1
Identify the nature of each compound in the pair: polar or nonpolar.
a. Both CH_3(CH_2)_3CH_3 and CH_3(CH_2)_5CH_3 are nonpolar hydrocarbons, so they will form a homogeneous solution due to dispersion forces.
b. CBr_4 is nonpolar, while H_2O is polar. These will not form a homogeneous solution due to the lack of common intermolecular forces.
c. LiNO_3 is an ionic compound, and H_2O is polar. They will form a homogeneous solution due to ion-dipole interactions.
d. CH_3OH is polar, and CH_3(CH_2)_3CH_3 is nonpolar. They will not form a homogeneous solution due to differing intermolecular forces.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Polarity
Polarity refers to the distribution of electrical charge over the atoms in a molecule. Polar molecules have a significant difference in electronegativity between their atoms, leading to partial positive and negative charges. This property influences solubility, as polar substances tend to dissolve well in polar solvents (like water), while nonpolar substances dissolve in nonpolar solvents (like hydrocarbons). Understanding polarity is crucial for predicting whether two compounds will form a homogeneous solution.
Recommended video:
Guided course
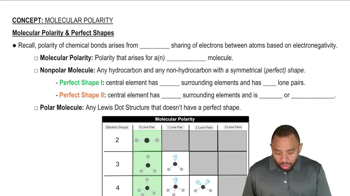
Molecular Polarity
Intermolecular Forces
Intermolecular forces are the forces of attraction or repulsion between neighboring particles (atoms, molecules, or ions). These forces include hydrogen bonding, dipole-dipole interactions, and London dispersion forces. The strength and type of these forces determine the solubility of compounds in one another; for example, hydrogen bonds can significantly enhance the solubility of polar compounds in water. Recognizing these forces helps in understanding the interactions that lead to homogeneous solutions.
Recommended video:
Guided course
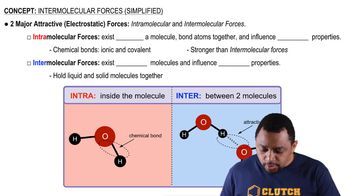
Intermolecular vs Intramolecular Forces
Homogeneous Solutions
A homogeneous solution is a mixture in which the components are uniformly distributed, resulting in a single phase. In chemistry, this often refers to solutions where solutes are completely dissolved in solvents, leading to consistent properties throughout the mixture. The formation of a homogeneous solution depends on the compatibility of the solute and solvent, which is influenced by their molecular characteristics, such as polarity and intermolecular forces. Identifying whether a pair of compounds forms a homogeneous solution is essential for understanding their chemical behavior.
Recommended video:
Guided course
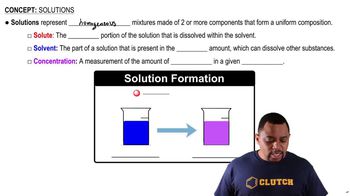
Solution Components
Related Practice
Textbook Question
Textbook Question
Determine whether each pair of compounds forms a homogeneous solution when combined. For those that form homogeneous solutions, indicate the type of forces that are involved. a. CCl4 and H2O b. KCl and H2O c. Br2 and CCl4
Textbook Question
Determine whether each pair of compounds forms a homogeneous solution when combined. For those that form homogeneous solutions, indicate the type of forces that are involved. d. CH3CH2OH and H2O
Textbook Question
Which compound would you expect to have greater surface tension: acetone [(CH3)2CO] or water (H2O)? Explain.
Textbook Question
The structures of two isomers of heptane are shown. Which of these two compounds would you expect to have the greater viscosity?
