Textbook Question
For a given type of MO, use a s2s as an example, is the bonding or antibonding orbital higher in energy? Explain.

 McMurry 8th Edition
McMurry 8th Edition Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure
Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure Problem 102
Problem 102 Verified step by step guidance
Verified step by step guidance

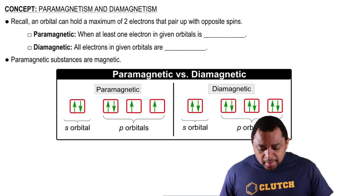

At high temperatures, sulfur vapor is predominantly in the form of S2(g) molecules. (a) Assuming that the molecular orbitals for third-row diatomic molecules are analogous to those for second-row molecules, construct an MO diagram for the valence orbitals of S2(g).
At high temperatures, sulfur vapor is predominantly in the form of S2(g) molecules. (d) When two electrons are added to S2, the disulfide ion S22- is formed. Is the bond length in S22- likely to be shorter or longer than the bond length in S2? Explain.