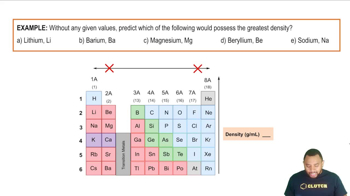
Trends in the Periodic Table
The periodic table exhibits specific trends in properties such as ionization energy, electronegativity, and atomic radius. As you move from left to right across a period, ionization energy tends to increase due to the increasing nuclear charge. Conversely, as you move down a group, ionization energy decreases because of the increased distance between the nucleus and the outermost electrons, which are more shielded by inner electrons.

 McMurry 8th Edition
McMurry 8th Edition Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory
Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory Problem 61a
Problem 61a Verified step by step guidance
Verified step by step guidance