Ch.5 - Periodicity & Electronic Structure of Atoms
 Back
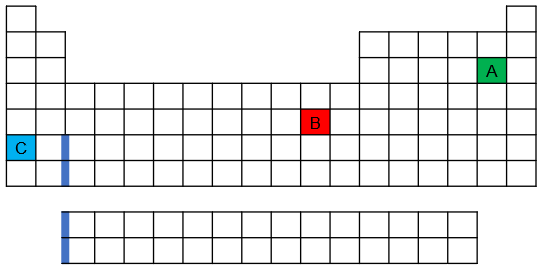
Back- One of the elements shown on the following periodic table has an anomalous ground-state electron configuration. Which is it—red, blue, or green—and why?
Problem 30
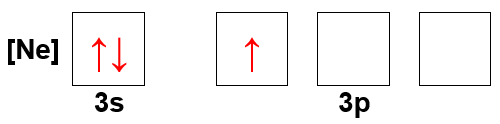
- What atom has the following orbital-filling diagram?
Problem 31
Problem 33
Which of the following three spheres represents a Ca atom, which an Sr atom, and which a Br atom?

- Which has the higher frequency, red light or violet light? Which has the longer wavelength? Which has the greater energy?
Problem 34
- Which has the higher frequency, infrared light or ultraviolet light? Which has the longer wavelength? Which has the greater energy?
Problem 35
- The Hubble Space Telescope detects electromagnetic energy in the wavelength range 1.15 * 10-7 m to 2.0 * 10-6 m. What region of the electromagnetic spectrum is found completely within this range? What regions fall partially in this range?
Problem 36
- The Green Bank Telescope in West Virginia—the world's largest steerable radio telescope—detects frequencies from 290 MHz to 90 GHz. What region or regions of the electro-magnetic spectrum are found completely or partially within its detection range?
Problem 37
- What is the frequency of a microwave with l = 4.33 * 10-3 m?
Problem 39
- A certain cellular telephone transmits at a frequency of 825 MHz and receives at a frequency of 875 MHz. (a) What is the wavelength of the transmitted signal in cm?
Problem 40
- (b) Fiber optic cable is available in 12 km lengths. How long will it take for a signal to travel that distance assuming that the speed of light in the cable is the same as in a vacuum?
Problem 41
- Calculate the energies of the following waves in kilojoules per mole, and tell which member of each pair has the higher value. (a) An FM radio wave at 99.5 MHz and an AM radio wave at 1150 kHz
Problem 42
- What is the energy of each of the following photons in kilojoules per mole? (a) v = 5.97 * 1019 s-1 (b) v = 1.26 * 106 s-1 (c)
Problem 45
= 2.57 * 102 m - (c) What type of electromagnetic radiation are these photons?
Problem 46
- (c) What is the color of the light with
Problem 47
= 450 nm? - The work function of cesium metal is 188 kJ/mol, which corresponds to light with a wavelength of 637 nm. Which of the following will cause the smallest number of electrons to be ejected from cesium? (a) High-amplitude wave with a wavelength of 500 nm (b) Low-amplitude wave with a wavelength of 500 nm (c) High-amplitude wave with a wavelength of 650 nm (d) Low-amplitude wave with a wavelength of 650 nm
Problem 52
- The work function of calcium metal is kJ/mol, which corresponds to light with a wavelength of 432 nm. Which of the following will cause the largest number of electrons to be ejected from cesium? (a) High-amplitude wave with a wavelength of 400 nm (b) Low-amplitude wave with a wavelength of 400 nm (c) High-amplitude wave with a wavelength of 450 nm (d) Low-amplitude wave with a wavelength of 450 nm
Problem 53
- The work function of silver metal is 436 kJ/mol. What frequency of light is needed to eject electrons from a sample of silver?
Problem 54
- Cesium metal is frequently used in photoelectric cells because the amount of energy necessary to eject electrons from a cesium surface is relatively small—only 206.5 kJ/mol. What wavelength of light in nanometers does this correspond to?
Problem 55
- Spectroscopy is a technique that uses the interaction of radiant energy with matter to identify or quantify a substance in a sample. A deuterium lamp is often used a light source in the ultraviolet region of the spectrum and the emission spectrum is shown. Is this a continuous or line emission spectrum?
Problem 56

- Sodium-vapor lamps are a common source of lighting. The emission spectrum from this type of lamp is shown. Is this a continuous or line emission spectrum?
Problem 57

- According to the equation for the Balmer line spectrum of hydrogen, a value of n = 3 gives a red spectral line at 656.3 nm, a value of n = 4 gives a green line at 486.1 nm, and a value of n = 5 gives a blue line at 434.0 nm. Calculate the energy in kilojoules per mole of the radiation corresponding to each of these spectral lines.
Problem 58
- Calculate the wavelength and energy in kilojoules necessary to completely remove an electron from the second shell (m = 2) of a hydrogen atom (R∞ = 1.097 * 10-2 nm-1).
Problem 61
- Use the Balmer equation to calculate the wavelength in nano-meters of the spectral line for hydrogen when n = 6 and m = 2. What is the energy in kilojoules per mole of the radiation corresponding to this line?
Problem 64
- Protons and electrons can be given very high energies in particle accelerators. What is the wavelength in meters of an electron (mass = 9.11 * 10-31 kg) that has been accelerated to 5% of the speed of light? In what region of the electromagnetic spectrum is this wavelength?
Problem 66
- What is the de Broglie wavelength in meters of a baseball weighing 145 g and traveling at 156km/h? Why do we not observe this wavelength?
Problem 68
- At what speed in meters per second must a 145 g baseball be traveling to have a de Broglie wavelength of 0.500 nm?
Problem 70
- What velocity would an electron (mass = 9.11 * 10-31 kg) need for its de Broglie wavelength to be that of red light (750 nm)?
Problem 71
- Use the Heisenberg uncertainty principle to calculate the uncertainty in meters in the position of a honeybee weighing 0.68 g and traveling at a velocity of 0.85 m/s. Assume that the uncertainty in the velocity is 0.1 m/s.
Problem 72
- The mass of a helium atom is 4.0026 amu, and its average velocity at 25 °C is 1.36 * 103 m/s. What is the uncertainty in meters in the position of a helium atom if the uncertainty in its velocity is 1%?
Problem 73
- What is the Heisenberg uncertainty principle, and how does it affect our description of atomic structure?
Problem 74
