Ch.23 - Organic and Biological Chemistry

Chapter 23, Problem 59
Give a line drawing and molecular formula for the following: (a) A linear alkane with four carbon atoms, (b) An alkene with six carbon atoms in a ring and one double bond, (c) A linear alkyne with six carbon atoms and two triple bonds.
 Verified step by step guidance
Verified step by step guidance1
Identify the structure of a linear alkane with four carbon atoms. Alkanes are saturated hydrocarbons with single bonds only. For four carbon atoms, the structure is a straight chain: C-C-C-C. The molecular formula is C_4H_10.
For an alkene with six carbon atoms in a ring and one double bond, recognize that this is a cycloalkene. A common example is cyclohexene, which has a six-membered carbon ring with one double bond. The molecular formula is C_6H_10.
Consider a linear alkyne with six carbon atoms and two triple bonds. Alkynes are unsaturated hydrocarbons with at least one carbon-carbon triple bond. For two triple bonds, the structure is linear: C≡C-C≡C-C-C. The molecular formula is C_6H_6.
Draw the line structure for each compound. For the alkane, draw a straight line with four vertices representing the carbon atoms. For the cycloalkene, draw a hexagon with one double bond. For the alkyne, draw a straight line with two triple bonds.
Verify the molecular formulas by counting the number of hydrogen atoms needed to satisfy the valency of carbon (four bonds per carbon atom). Ensure the molecular formulas match the structures drawn.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Alkanes
Alkanes are saturated hydrocarbons consisting only of carbon (C) and hydrogen (H) atoms, connected by single bonds. They follow the general formula CnH2n+2, where 'n' is the number of carbon atoms. For example, a linear alkane with four carbon atoms is butane (C4H10), which can be represented in a line drawing showing the carbon backbone with hydrogen atoms attached.
Recommended video:
Guided course
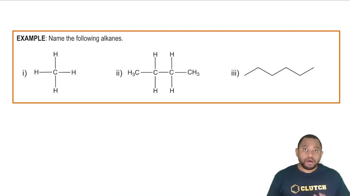
Naming Alkanes Example
Alkenes
Alkenes are unsaturated hydrocarbons that contain at least one carbon-carbon double bond. They follow the general formula CnH2n, indicating that they have fewer hydrogen atoms than alkanes due to the presence of double bonds. A cyclic alkene with six carbon atoms and one double bond can be represented as cyclohexene (C6H10), where the double bond is depicted in the ring structure.
Recommended video:
Guided course
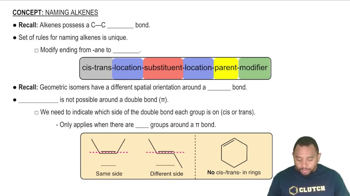
Rules for Naming Alkenes
Alkynes
Alkynes are unsaturated hydrocarbons characterized by one or more carbon-carbon triple bonds. They follow the general formula CnH2n-2, which reflects the reduction in hydrogen atoms due to the triple bonds. A linear alkyne with six carbon atoms and two triple bonds can be represented as 1,5-hexadiyne (C6H6), where the structure shows the carbon chain with the appropriate placement of triple bonds.
Recommended video:
Guided course
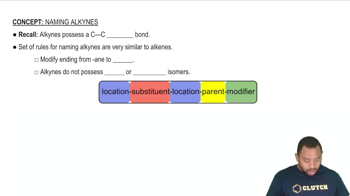
Rules for Naming Alkynes
Related Practice
