Which of the group 4A elements have allotropes with the diamond structure? Which have metallic allotropes? How does the variation in the structure of the group 4A elements illustrate how metallic character varies down a periodic group?
Ch.22 - The Main Group Elements

Chapter 22, Problem 22.3
Which of the following elements (X) will form a covalent hydride with the formula XH3 that is a gas at room temperature? (LO 22.4)
(a) Al (b) As (c) Ba (d) Se
 Verified step by step guidance
Verified step by step guidance1
Identify the elements that can form covalent bonds with hydrogen to create a hydride.
Consider the periodic table position of each element: Al, As, Ba, and Se.
Recall that covalent hydrides are typically formed by nonmetals or metalloids.
Determine which of the given elements is likely to form a gaseous hydride at room temperature.
Recognize that As (arsenic) is a metalloid that can form a covalent hydride, AsH_3, which is a gas at room temperature.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Covalent Bonding
Covalent bonding occurs when two atoms share one or more pairs of electrons, typically between nonmetals. This type of bond results in the formation of molecules, such as hydrides, where the hydrogen atom bonds with another element. Understanding the nature of covalent bonds is essential for predicting the properties of compounds, including their state at room temperature.
Recommended video:
Guided course
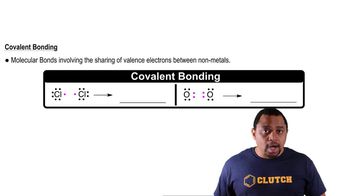
Chemical Bonds
Hydrides
Hydrides are compounds formed between hydrogen and another element. They can be classified into ionic and covalent hydrides, with covalent hydrides being formed by nonmetals. The physical state of hydrides at room temperature can vary; for instance, covalent hydrides of lighter nonmetals tend to be gases, while those of heavier elements may be liquids or solids.
Recommended video:
Guided course
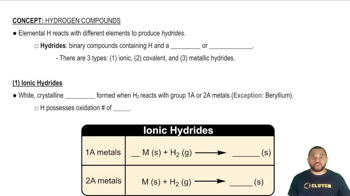
Ionic Hydrides
Periodic Trends
Periodic trends refer to the predictable patterns in the properties of elements as you move across or down the periodic table. These trends influence factors such as electronegativity, atomic size, and the ability to form covalent bonds. Recognizing these trends helps in determining which elements are likely to form gaseous hydrides, as lighter elements in specific groups tend to form such compounds.
Recommended video:
Guided course

Periodic Trends
Related Practice
Textbook Question
Textbook Question
GeCl4 reacts with Cl- to give GeCl62-, but CCl4 does not react with excess Cl-. Explain.
Textbook Question
Using the shorthand notation of Figure 22.9, draw the structure of the cyclic silicate anion in which four SiO4 tetrahedra share O atoms to form an eight-membered ring of alternating Si and O atoms. Give the formula and charge of the anion.
Textbook Question
Compare and contrast the properties of ammonia and phosphine.
Textbook Question
Give an example of an ionic carbide. What is the oxidation state of carbon in this substance?
Textbook Question
Draw the electron-dot structure for CO, CO2, and CO32–, and predict which substance will have the strongest carbon–oxygen bond.
