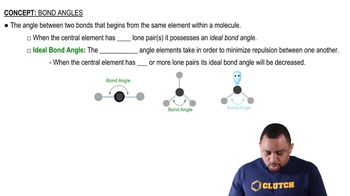
Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. It is determined by the number of bonding pairs and lone pairs of electrons around the central atom, which influences the overall shape, such as linear, bent, or tetrahedral. For H2Se, understanding its molecular geometry helps in predicting its physical and chemical properties.

 Verified step by step guidance
Verified step by step guidance