Ch.22 - The Main Group Elements

Chapter 22, Problem 44
Consider the elements C, Se, B, Sn, and Cl. Identify which of these elements: (a) Has a maximum oxidation state of +6, (b) Has the largest atomic radius, (c) Is the most electronegative.
 Verified step by step guidance
Verified step by step guidance1
Step 1: To determine which element has a maximum oxidation state of +6, consider the common oxidation states of each element. Carbon (C) can have a maximum oxidation state of +4, Selenium (Se) can have a maximum oxidation state of +6, Boron (B) typically has a maximum oxidation state of +3, Tin (Sn) can have a maximum oxidation state of +4, and Chlorine (Cl) can have a maximum oxidation state of +7. Therefore, identify the element with a maximum oxidation state of +6.
Step 2: To find the element with the largest atomic radius, consider the position of each element in the periodic table. Atomic radius generally increases down a group and decreases across a period. Compare the positions of Carbon (C), Selenium (Se), Boron (B), Tin (Sn), and Chlorine (Cl) to determine which has the largest atomic radius.
Step 3: To identify the most electronegative element, use the periodic trend that electronegativity increases across a period and decreases down a group. Compare the electronegativities of Carbon (C), Selenium (Se), Boron (B), Tin (Sn), and Chlorine (Cl) to find the most electronegative element.
Step 4: Review the periodic table trends: Electronegativity and atomic radius are key periodic trends. Electronegativity increases from left to right across a period and decreases down a group, while atomic radius decreases from left to right across a period and increases down a group.
Step 5: Summarize your findings: Based on the analysis of oxidation states, atomic radii, and electronegativities, identify the elements that meet the criteria for each part of the problem.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Oxidation States
Oxidation states indicate the degree of oxidation of an atom in a compound, reflecting the number of electrons lost or gained. Elements can exhibit multiple oxidation states, with the maximum oxidation state being the highest positive charge an element can achieve. For example, carbon can have oxidation states ranging from -4 to +4, while elements like sulfur can reach +6.
Recommended video:
Guided course

Oxidation Numbers
Atomic Radius
The atomic radius is a measure of the size of an atom, typically defined as the distance from the nucleus to the outermost electron shell. Atomic radius generally increases down a group in the periodic table due to the addition of electron shells, while it decreases across a period from left to right due to increased nuclear charge. This concept is crucial for comparing the sizes of elements like Sn and Cl.
Recommended video:
Guided course
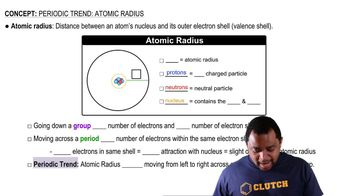
Atomic Radius
Electronegativity
Electronegativity is a measure of an atom's ability to attract and hold onto electrons in a chemical bond. It is influenced by the atomic radius and the effective nuclear charge experienced by the valence electrons. The most electronegative element in the periodic table is fluorine, but among the given elements, chlorine is the most electronegative, making it essential for understanding chemical reactivity.
Recommended video:
Guided course

Electronegativity Trends
Related Practice
