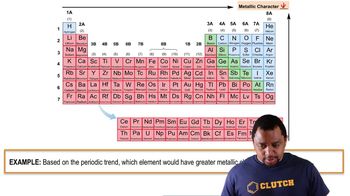
Anions and Their Formation
Anions are negatively charged ions formed when an atom gains one or more electrons. Nonmetals, particularly those in groups 15, 16, and 17 of the periodic table, commonly form anions. For example, sulfur (S) can gain two electrons to form a 2- anion (S^2-), which is essential for understanding the reactivity and bonding behavior of elements.

 Verified step by step guidance
Verified step by step guidance