Predict the crystal field energy-level diagram for a linear ML2 complex that has two ligands along the :

 McMurry 8th Edition
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.114
Problem 21.114Which of the following complexes are paramagnetic?
(a) [Mn(CN)6]3-
(b) [Zn(NH3)4]2+ (tetrahedral)
(c) [Fe(CN)6]4-
(d) [FeF6]4-
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
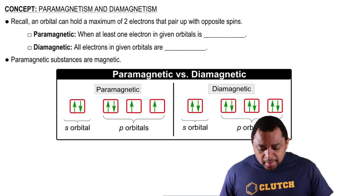
Paramagnetism
Crystal Field Theory

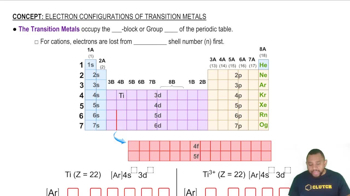
Oxidation States of Transition Metals
Based on the wavelength of maximum absorption of the cobalt complexes, arrange the following ligands in a spectrochemical series from weakest-field to strongest-field ligand.
(a) Cl- < NCS- < H2O < NH3
(b) Cl- < NCS- < H2O < NH3
(c) H2O < Cl- < NH3 < NCS-
(d) Cl- < H2O < NCS- < NH3
For each of the following complexes, draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons.
(a) [CrF6]3-
(b) [V(H2O)6]3+
(c) [Fe(CN)6]3-
Draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons for each of the following.
(a) [Cu(en)3]2+
(b) [FeF6]2-
(c) [Co(en)3]3+ (low spin)
What is the systematic name for each of the following coordination compounds?
(c) [Co(NH3)4Br2]Br
(d) Cu(gly)2
Look at the colors of the isomeric complexes in Figure 21.12, and predict which is the stronger field ligand, nitro (-NO2) of nitrito (-ONO). Explain.