Predict the crystal field energy-level diagram for a square pyramidal ML5 complex that has two ligands along the axes but only one ligand along the z axis. Your diagram should be intermediate between those for an octahedral ML6 complex and a square planar ML4 complex.
Ch.21 - Transition Elements and Coordination Chemistry

Chapter 21, Problem 21.95
The glycinate anion, gly-= NH2CH2CO2 -, bonds to metal ions through the N atom and one of the O atoms. Using to represent gly-, sketch the structures of the four stereoisomers of Co(gly)3.
 Verified step by step guidance
Verified step by step guidance1
Identify the coordination geometry of the complex. Cobalt(III) typically forms octahedral complexes.
Recognize that each glycinate ligand is bidentate, meaning it can form two bonds with the metal center, one through the nitrogen atom and one through an oxygen atom.
Consider the possible arrangements of the three bidentate ligands around the cobalt center. In an octahedral complex, these can lead to different stereoisomers.
Determine the possible stereoisomers: In an octahedral complex with three bidentate ligands, there are two main types of stereoisomers: facial (fac) and meridional (mer).
Sketch the structures: For the fac isomer, all three ligands are adjacent to each other, forming a face of the octahedron. For the mer isomer, the ligands are arranged such that they form a meridian around the metal center.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Stereoisomerism
Stereoisomerism refers to the phenomenon where compounds have the same molecular formula and connectivity of atoms but differ in the spatial arrangement of those atoms. In coordination chemistry, stereoisomers can arise from the different ways ligands can be arranged around a central metal ion, leading to distinct geometric configurations such as cis and trans forms.
Recommended video:
Guided course

Stereoisomers
Coordination Complexes
Coordination complexes consist of a central metal atom or ion bonded to surrounding molecules or anions called ligands. The nature of these bonds and the arrangement of ligands around the metal ion determine the properties and reactivity of the complex. In this case, the glycinate anion acts as a bidentate ligand, coordinating through both nitrogen and oxygen atoms.
Recommended video:
Guided course
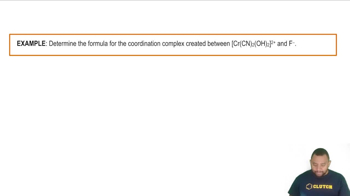
Coordination Complexes Example
Chirality
Chirality is a property of a molecule that makes it non-superimposable on its mirror image, much like left and right hands. In coordination complexes, chirality can arise when a metal center is bonded to ligands in such a way that it creates asymmetric arrangements. The presence of chiral ligands, like glycinate, can lead to the formation of enantiomers, which are important in biological systems.
Recommended video:
Guided course
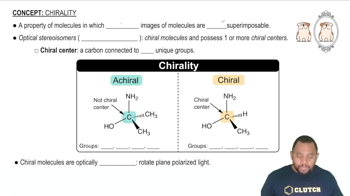
Chirality
Related Practice
Textbook Question
Textbook Question
Although Cl- is a weak-field ligand and CN- is a strong field ligand, [CrCl6]3- and [Cr(CN)6]3- exhibit approximately the same amount of paramagnetism. Explain.
Textbook Question
The Ni2+(aq) cation is green, but Zn2+(aq) is colorless. Explain.
Textbook Question
What is the oxidation state of the metal in each of the complexes?
a. [Ni(CN)5]3–
b. Ni(CO)4
c. [Co(en)2(H2O)Br]2+
d. [Cu(H2O)2(C2O4)2]2–
e. Co(NH3)3(NO2)3
Textbook Question
Explain why [CoCl4]2- (blue) and [Co(H2O)6]2+ (pink) have different colors. Which complex has its absorption bands at longer wavelengths?
Textbook Question
Draw all possible diastereoisomers of [Cr(C2O4)2(H2O)2]-. Which can exist as a pair of enantiomers?
