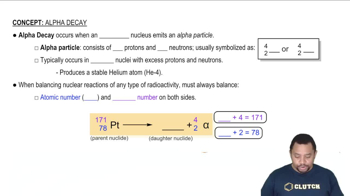
Textbook Question
Radioactive decay exhibits a first-order rate law, rate = -∆N/∆t = kN, where N denotes the number of radio-active nuclei present at time t. The half-life of strontium-90, a dangerous nuclear fission product, is 29 years.(a) What fraction of the strontium-90 remains after three half-lives?