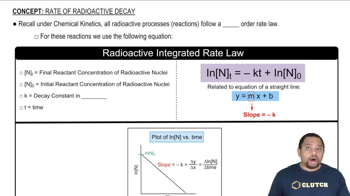
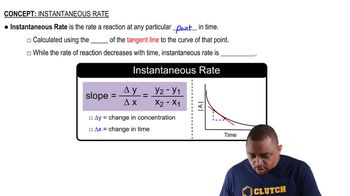
Molar Mass and Avogadro's Number
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). For potassium chloride (KCl), the molar mass can be calculated by adding the atomic masses of potassium (K) and chlorine (Cl). Avogadro's number (6.022 x 10^23) is the number of particles in one mole of a substance, enabling the conversion between grams and the number of ions or molecules.

 Verified step by step guidance
Verified step by step guidance