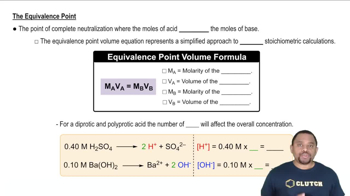
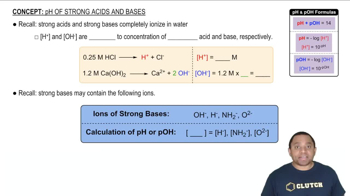
Textbook Question
Consider the titration of 40.0 mL of 0.250 M HF with 0.200 M NaOH. How many milliliters of base are required to reach the equivalence point? Calculate the pH at each of the following points. (d) After the addition of 80.0 mL of base