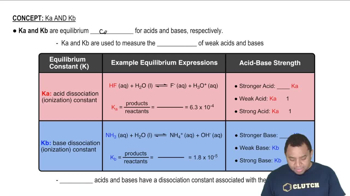
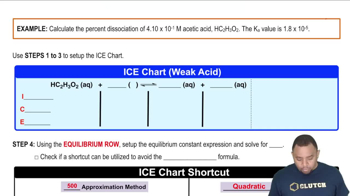
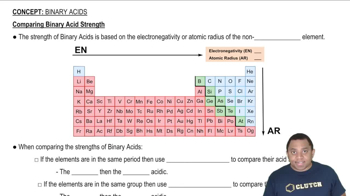
Acid Dissociation Constant (Ka)
The acid dissociation constant, Ka, quantifies the strength of an acid in solution. It is defined as the equilibrium constant for the dissociation of an acid into its conjugate base and a proton. A higher Ka value indicates a stronger acid, which dissociates more completely in solution, leading to a greater concentration of hydrogen ions.

 Verified step by step guidance
Verified step by step guidance