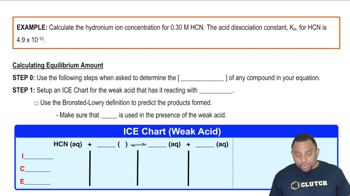
Textbook Question
Consider the reaction: SO2 + OH- S HSO3-. Which reaction scheme shows the correct use of the curved arrow notation representing thedonation of an electron pair and the correct labeling of the Lewis acid and Lewis base? (LO 16.14)(a) (b) (c) (d)