Textbook Question
How would you prepare each of the following solutions? (a) 100 mL of a 155 ppm solution of urea, CH4N2O, in water

 Verified step by step guidance
Verified step by step guidance
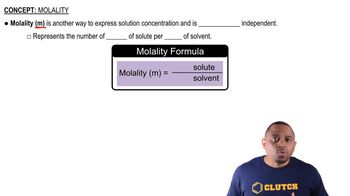

How would you prepare each of the following solutions? (b) 100 mL of an aqueous solution whose K+ concentration is 0.075 M
How would you prepare 165 mL of a 0.0268 M solution of benzoic acid 1C7H6O22 in chloroform 1CHCl32?
Which of the following solutions is more concentrated? (a) 0.500 M KCl or 0.500 mass % KCl in water