Textbook Question
How would you prepare each of the following solutions?(c) A solution of methyl alcohol (methanol) and water in which Xmethanol = 0.15 and Xwater = 0.85

 Verified step by step guidance
Verified step by step guidance

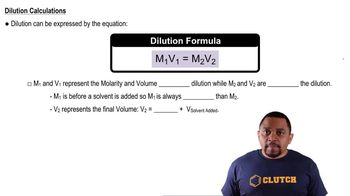

How would you prepare each of the following solutions? (b) 100 mL of an aqueous solution whose K+ concentration is 0.075 M
Which of the following solutions is more concentrated? (a) 0.500 M KCl or 0.500 mass % KCl in water