Chlorine reacts with oxygen to form Cl2O7. (b) Write a balanced equation for the formation of Cl2O71l2 from the elements.
Ch.7 - Periodic Properties of the Elements

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 7, Problem 64a
An element X reacts with oxygen to form XO2 and with chlorine to form XCl4. XO2 is a white solid that melts at high temperatures (above 1000 °C). Under usual conditions, XCl4 is a colorless liquid with a boiling point of 58 °C. (a) XCl4 reacts with water to form XO2 and another product. What is the likely identity of the other product?
 Verified step by step guidance
Verified step by step guidance1
Identify the reaction between XCl4 and water. The given information states that XO2 and another product are formed.
Write the chemical equation for the reaction of XCl4 with water. Since XO2 is one of the products and XCl4 contains chlorine, hypothesize that the other product involves chlorine.
Consider the common reactions of chlorine in compounds when they react with water. Typically, chlorine can form hydrochloric acid (HCl).
Propose a balanced chemical equation: XCl4 + H2O → XO2 + 4 HCl. This equation suggests that hydrochloric acid (HCl) is the other product formed.
Verify the stoichiometry of the proposed reaction to ensure mass and charge are balanced, confirming that all atoms on the reactants side are accounted for in the products.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Reactions
Chemical reactions involve the transformation of reactants into products through the breaking and forming of chemical bonds. In this case, the reaction of XCl4 with water illustrates a hydrolysis reaction, where water reacts with a compound to produce new substances, including an oxide and an acid or another product.
Recommended video:
Guided course

Chemical Properties
Oxides and Halides
Oxides are compounds formed by the reaction of an element with oxygen, while halides are compounds formed with halogens like chlorine. The question indicates that X forms XO2 (an oxide) and XCl4 (a halide), which suggests that X is likely a non-metal that can form stable compounds with both oxygen and chlorine.
Recommended video:
Guided course
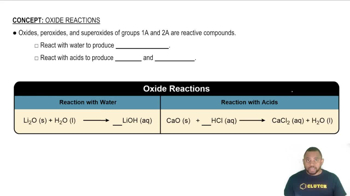
Oxide Reactions
Acid-Base Reactions
Acid-base reactions involve the transfer of protons (H+) between reactants. In the context of the reaction between XCl4 and water, the likely other product is an acid formed from the chlorine component, such as hydrochloric acid (HCl), indicating that XCl4 acts as a Lewis acid in this reaction.
Recommended video:
Guided course

Acid-Base Reaction
Related Practice
Textbook Question
Textbook Question
Chlorine reacts with oxygen to form Cl2O7. (a) What is the name of this product (see Table 2.6)?
1
views
Textbook Question
Chlorine reacts with oxygen to form Cl2O7. (c) Would you expect Cl2O7 to be more reactive toward H+1aq2 or OH-1aq2?
Textbook Question
An element X reacts with oxygen to form XO2 and with chlorine to form XCl4. XO2 is a white solid that melts at high temperatures (above 1000 °C). Under usual conditions, XCl4 is a colorless liquid with a boiling point of 58 °C. (b) Do you think that element X is a metal, nonmetal, or metalloid?
Textbook Question
Write balanced equations for the following reactions. a. barium oxide with water
Textbook Question
Write balanced equations for the following reactions. c. sulfur trioxide with water
