Chlorine reacts with oxygen to form Cl2O7. (b) Write a balanced equation for the formation of Cl2O71l2 from the elements.
Ch.7 - Periodic Properties of the Elements

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 7, Problem 63a
Chlorine reacts with oxygen to form Cl2O7. (a) What is the name of this product (see Table 2.6)?
 Verified step by step guidance
Verified step by step guidance1
Identify the type of compound formed by chlorine and oxygen.
Recognize that Cl2O7 is a molecular compound composed of nonmetals.
Use the rules for naming binary molecular compounds: the first element is named first, and the second element is named as if it were an anion.
For the second element, use the root of the element name and add the suffix '-ide'.
Use prefixes to indicate the number of atoms of each element: 'di-' for two chlorine atoms and 'hepta-' for seven oxygen atoms.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Nomenclature
Chemical nomenclature is the system of naming chemical compounds based on their composition and structure. It follows specific rules set by organizations like the International Union of Pure and Applied Chemistry (IUPAC). Understanding nomenclature helps in identifying compounds and their properties, which is essential for answering questions about chemical reactions.
Recommended video:
Guided course

Chemical Properties
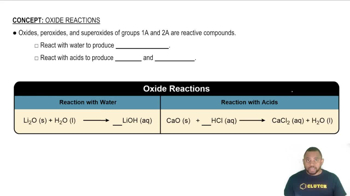
Oxides
Oxides are compounds formed when elements react with oxygen. They can be classified into various types, such as metal oxides and non-metal oxides, depending on the elements involved. In this case, Cl2O7 is a non-metal oxide, specifically a dichlorine heptoxide, which is important for understanding the nature of the product formed in the reaction.
Recommended video:
Guided course
Oxide Reactions
Stoichiometry
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. It allows chemists to predict the amounts of substances consumed and produced in a reaction. While the question focuses on naming the product, understanding stoichiometry is crucial for grasping the overall reaction dynamics and the significance of the compound formed.
Recommended video:
Guided course

Stoichiometry Concept
Related Practice
Textbook Question
Textbook Question
Predict whether each of the following oxides is ionic or molecular: SnO2, Al2O3, CO2, Li2O, Fe2O3, H2O.
1
views
Textbook Question
Would you expect manganese(II) oxide, MnO, to react more readily with HCl(aq) or NaOH(aq)?
Textbook Question
Arrange the following oxides in order of increasing acidity: CO2,CaO,Al2O3,SO3,SiO2,P2O5.
Textbook Question
Chlorine reacts with oxygen to form Cl2O7. (c) Would you expect Cl2O7 to be more reactive toward H+1aq2 or OH-1aq2?
Textbook Question
An element X reacts with oxygen to form XO2 and with chlorine to form XCl4. XO2 is a white solid that melts at high temperatures (above 1000 °C). Under usual conditions, XCl4 is a colorless liquid with a boiling point of 58 °C. (a) XCl4 reacts with water to form XO2 and another product. What is the likely identity of the other product?
