In the experiment shown schematically below, a beam of neutral atoms is passed through a magnetic field. Atoms that have unpaired electrons are deflected in different directions in the magnetic field depending on the value of the electron spin quantum number. In the experiment illustrated, we envision that a beam of hydrogen atoms splits into two beams. (a) What is the significance of the observation that the single beam splits into two beams?
Ch.6 - Electronic Structure of Atoms

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 6, Problem 104
The stratospheric ozone (O3) layer helps to protect us from harmful ultraviolet radiation. It does so by absorbing ultraviolet light and falling apart into an O2 molecule and an oxygen atom, a process known as photodissociation. O3(g) → O2(g) + O(g). Use the data in Appendix C to calculate the enthalpy change for this reaction. What is the maximum wavelength a photon can have if it is to possess sufficient energy to cause this dissociation? In what portion of the spectrum does this wavelength occur?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the reaction and the enthalpy change needed. The reaction is O_3(g) \rightarrow O_2(g) + O(g). We need to calculate the enthalpy change (\Delta H) for this reaction using standard enthalpies of formation from Appendix C.
Step 2: Use the formula for the enthalpy change of a reaction: \Delta H_{reaction} = \sum \Delta H_f^{\circ} (products) - \sum \Delta H_f^{\circ} (reactants). Look up the standard enthalpies of formation for O_3(g), O_2(g), and O(g) in Appendix C.
Step 3: Substitute the values from Appendix C into the enthalpy change formula. Calculate \Delta H_{reaction} by subtracting the enthalpy of formation of O_3(g) from the sum of the enthalpies of formation of O_2(g) and O(g).
Step 4: To find the maximum wavelength of a photon that can cause this dissociation, use the energy-wavelength relationship: E = \frac{hc}{\lambda}, where E is the energy required (equal to \Delta H_{reaction} per mole of O_3), h is Planck's constant, c is the speed of light, and \lambda is the wavelength.
Step 5: Solve for \lambda (wavelength) by rearranging the equation: \lambda = \frac{hc}{E}. Calculate \lambda using the energy value obtained from \Delta H_{reaction}. Determine the portion of the electromagnetic spectrum this wavelength falls into by comparing it to known ranges (e.g., ultraviolet, visible, etc.).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Photodissociation
Photodissociation is a process in which a chemical compound breaks down into simpler molecules or atoms upon absorbing light, typically ultraviolet (UV) radiation. In the case of ozone (O3), it absorbs UV light, leading to its dissociation into oxygen molecules (O2) and oxygen atoms (O). This reaction is crucial for understanding how ozone protects the Earth from harmful UV radiation.
Enthalpy Change
Enthalpy change (ΔH) refers to the heat content change of a system at constant pressure during a chemical reaction. It can be calculated using standard enthalpy values from tables, which provide the energy associated with the formation or dissociation of compounds. In this context, calculating the enthalpy change for the photodissociation of ozone helps to understand the energy dynamics involved in the reaction.
Recommended video:
Guided course

Enthalpy of Formation
Photon Energy and Wavelength
The energy of a photon is inversely related to its wavelength, described by the equation E = hc/λ, where E is energy, h is Planck's constant, c is the speed of light, and λ is the wavelength. To cause photodissociation, the photon must have sufficient energy to break the O3 bond, which can be determined by calculating the maximum wavelength that corresponds to this energy. This wavelength typically falls within the ultraviolet region of the electromagnetic spectrum.
Recommended video:
Guided course
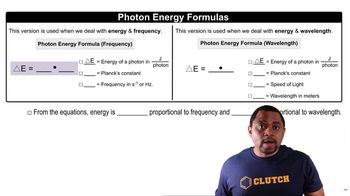
Photon Energy Formulas
Related Practice
Textbook Question
Textbook Question
In the experiment shown schematically below, a beam of neutral atoms is passed through a magnetic field. Atoms that have unpaired electrons are deflected in different directions in the magnetic field depending on the value of the electron spin quantum number. In the experiment illustrated, we envision that a beam of hydrogen atoms splits into two beams. (c) What do you think would happen if the beam of hydrogen atoms were replaced with a beam of helium atoms? Why?
