Scientists have speculated that element 126 might have a moderate stability, allowing it to be synthesized and characterized. Predict what the condensed electron configuration of this element might be.

Microwave ovens use microwave radiation to heat food. The energy of the microwaves is absorbed by water molecules in food and then transferred to other components of the food. (a) Suppose that the microwave radiation has a wavelength of 10 cm. How many photons are required to heat 200 mL of water from 25 to 75 °C?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Photon Energy
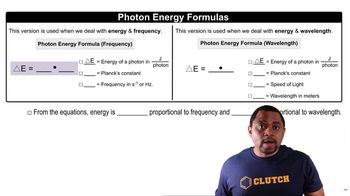
Specific Heat Capacity

Energy Transfer in Heating

In the experiment shown schematically below, a beam of neutral atoms is passed through a magnetic field. Atoms that have unpaired electrons are deflected in different directions in the magnetic field depending on the value of the electron spin quantum number. In the experiment illustrated, we envision that a beam of hydrogen atoms splits into two beams. (a) What is the significance of the observation that the single beam splits into two beams?
In the experiment shown schematically below, a beam of neutral atoms is passed through a magnetic field. Atoms that have unpaired electrons are deflected in different directions in the magnetic field depending on the value of the electron spin quantum number. In the experiment illustrated, we envision that a beam of hydrogen atoms splits into two beams. (c) What do you think would happen if the beam of hydrogen atoms were replaced with a beam of helium atoms? Why?
