Using the periodic table as a guide, write the condensed electron configuration and determine the number of unpaired electrons for the ground state of d. Sb
Ch.6 - Electronic Structure of Atoms

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 6, Problem 102a
In the experiment shown schematically below, a beam of neutral atoms is passed through a magnetic field. Atoms that have unpaired electrons are deflected in different directions in the magnetic field depending on the value of the electron spin quantum number. In the experiment illustrated, we envision that a beam of hydrogen atoms splits into two beams. (a) What is the significance of the observation that the single beam splits into two beams?

 Verified step by step guidance
Verified step by step guidance1
Identify the key components of the experiment: a beam of neutral hydrogen atoms, a slit, a horseshoe magnet, and a beam plate collector.
Understand that the hydrogen atoms have one electron, which can have an electron spin quantum number of either +1/2 or -1/2.
Recognize that the magnetic field created by the horseshoe magnet interacts with the magnetic moments of the unpaired electrons in the hydrogen atoms.
Explain that the interaction between the magnetic field and the electron spins causes the beam of hydrogen atoms to split into two separate beams, corresponding to the two possible spin states (+1/2 and -1/2).
Conclude that the observation of the beam splitting into two beams signifies the presence of unpaired electrons in the hydrogen atoms and demonstrates the quantization of electron spin.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
59sWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electron Spin Quantum Number
The electron spin quantum number (s) describes the intrinsic angular momentum of electrons, which can take values of +1/2 or -1/2. This property is crucial in determining how electrons behave in magnetic fields, as unpaired electrons will align with or against the magnetic field, leading to observable effects such as deflection in experiments.
Recommended video:
Guided course
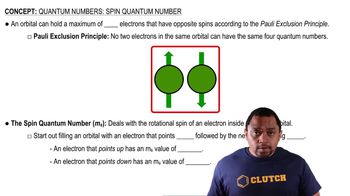
Spin Quantum Number
Magnetic Deflection of Atoms
When a beam of neutral atoms passes through a magnetic field, atoms with unpaired electrons experience a force due to their magnetic moments. This force causes the atoms to deflect in different directions based on their spin states, resulting in the splitting of the beam into distinct paths, which can be measured and analyzed.
Recommended video:
Guided course

Magnetic Quantum Example
Significance of Beam Splitting
The splitting of a single beam of hydrogen atoms into two distinct beams indicates the presence of two different spin states of the electrons in the atoms. This observation is significant as it provides evidence for quantum mechanical properties of particles, illustrating how quantum states can lead to observable macroscopic effects in experiments.
Recommended video:
Guided course

Significant Figures Example
Related Practice
Textbook Question
Textbook Question
Scientists have speculated that element 126 might have a moderate stability, allowing it to be synthesized and characterized. Predict what the condensed electron configuration of this element might be.
Textbook Question
In the experiment shown schematically below, a beam of neutral atoms is passed through a magnetic field. Atoms that have unpaired electrons are deflected in different directions in the magnetic field depending on the value of the electron spin quantum number. In the experiment illustrated, we envision that a beam of hydrogen atoms splits into two beams. (c) What do you think would happen if the beam of hydrogen atoms were replaced with a beam of helium atoms? Why?
