(b) What is the change in potential energy if the distance separating the two electrons is increased to 1.0 nm?

Use the equations given in Problem 5.15 to calculate: (a) The electrostatic force of repulsion for two electrons separated by 75 pm. (b) The gravitational force of attraction for two electrons separated by 75 pm. (c) If allowed to move, will the electrons be repelled or attracted to one another?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
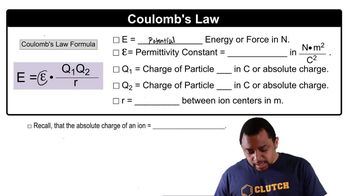
Coulomb's Law
Newton's Law of Universal Gravitation
Nature of Electric and Gravitational Forces

(c) Does the potential energy of the two particles increase or decrease when the distance is increased to 1.0 nm?
(a) The electrostatic force (not energy) of attraction between two oppositely charged objects is given by the equation F = k (Q1Q2/d2) where k = 8.99⨉109N-m2/C2, Q1 and Q2 are the charges of the two objects in Coulombs, and d is the distance separating the two objects in meters. What is the electrostatic force of attraction (in Newtons) between an electron and a proton that are separated by 1⨉102 pm?
A sodium ion, Na+, with a charge of 1.6⨉10-19 C and a chloride ion, Cl - , with charge of -1.6⨉10-19 C, are separated by a distance of 0.50 nm. How much work would be required to increase the separation of the two ions to an infinite distance?
A magnesium ion, Mg2+, with a charge of 3.2⨉10-19 C and an oxide ion, O2-, with a charge of -3.2⨉10-19 C, are separated by a distance of 0.35 nm. How much work would be required to increase the separation of the two ions to an infinite distance?
