(a) The electrostatic force (not energy) of attraction between two oppositely charged objects is given by the equation F = k (Q1Q2/d2) where k = 8.99⨉109N-m2/C2, Q1 and Q2 are the charges of the two objects in Coulombs, and d is the distance separating the two objects in meters. What is the electrostatic force of attraction (in Newtons) between an electron and a proton that are separated by 1⨉102 pm?
Ch.5 - Thermochemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 5, Problem 18
A magnesium ion, Mg2+, with a charge of 3.2⨉10-19 C and an oxide ion, O2-, with a charge of -3.2⨉10-19 C, are separated by a distance of 0.35 nm. How much work would be required to increase the separation of the two ions to an infinite distance?
 Verified step by step guidance
Verified step by step guidance1
Identify the charges of the ions involved. The magnesium ion, Mg2+, has a charge of +3.2⨉10-19 C, and the oxide ion, O2-, has a charge of -3.2⨉10-19 C.
Convert the distance from nanometers to meters. Since 1 nm = 1⨉10-9 meters, 0.35 nm is equivalent to 0.35⨉10-9 meters.
Use Coulomb's Law to calculate the initial electrostatic force between the ions. The formula for Coulomb's Law is F = k \frac{{|q1 \cdot q2|}}{{r^2}}, where k is Coulomb's constant (approximately 8.988⨉109 N⋅m2/C2), q1 and q2 are the charges of the ions, and r is the separation distance.
Calculate the initial potential energy using the formula U = k \frac{{q1 \cdot q2}}{{r}}, where U is the potential energy, and the other symbols have their usual meanings.
To find the work required to separate the ions to an infinite distance, calculate the change in potential energy, which is the difference between the final potential energy (zero, at infinite distance) and the initial potential energy.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Coulomb's Law
Coulomb's Law describes the electrostatic force between two charged particles. It states that the force is directly proportional to the product of the magnitudes of the charges and inversely proportional to the square of the distance between them. This principle is essential for calculating the work done in moving charged particles, as it helps determine the force acting on them at different separations.
Recommended video:
Guided course
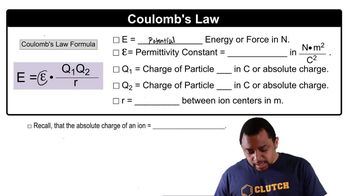
Coulomb's Law Concept 2
Electric Potential Energy
Electric potential energy is the energy stored in a system of charged particles due to their positions relative to each other. It can be calculated using the formula U = k * (q1 * q2) / r, where U is the potential energy, k is Coulomb's constant, q1 and q2 are the charges, and r is the distance between them. Understanding this concept is crucial for determining the work required to separate the ions to an infinite distance.
Recommended video:
Guided course
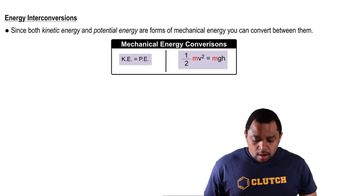
Kinetic & Potential Energy
Work-Energy Principle
The Work-Energy Principle states that the work done on an object is equal to the change in its energy. In the context of charged particles, the work required to move them from one position to another can be calculated by finding the difference in electric potential energy at those positions. This principle allows us to quantify the energy needed to increase the separation of the magnesium and oxide ions to infinity.
Recommended video:
Guided course
Uncertainty Principle Formula
Related Practice
Textbook Question
Textbook Question
A sodium ion, Na+, with a charge of 1.6⨉10-19 C and a chloride ion, Cl - , with charge of -1.6⨉10-19 C, are separated by a distance of 0.50 nm. How much work would be required to increase the separation of the two ions to an infinite distance?
Textbook Question
Predict the chemical formula of the ionic compound formed between the following pairs of elements: (a) Al and F
Textbook Question
(a) According to the first law of thermodynamics, what quantity is conserved?
2
views
