(a) What is the electrostatic potential energy (in joules) between two electrons that are separated by 62 pm?
Ch.5 - Thermochemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 5, Problem 15a
(a) The electrostatic force (not energy) of attraction between two oppositely charged objects is given by the equation F = k (Q1Q2/d2) where k = 8.99⨉109N-m2/C2, Q1 and Q2 are the charges of the two objects in Coulombs, and d is the distance separating the two objects in meters. What is the electrostatic force of attraction (in Newtons) between an electron and a proton that are separated by 1⨉102 pm?
 Verified step by step guidance
Verified step by step guidance1
Convert the distance from picometers to meters. Since 1 pm = 1⨉10^{-12} m, multiply 1⨉10^{2} pm by 1⨉10^{-12} m/pm to get the distance in meters.
Identify the charges of the electron and proton. The charge of an electron (Q_1) is -1.6⨉10^{-19} C, and the charge of a proton (Q_2) is +1.6⨉10^{-19} C.
Substitute the values of Q_1, Q_2, and the converted distance (d) into the formula F = k (Q_1Q_2/d^2).
Use the given value of k = 8.99⨉10^{9} N-m^2/C^2 in the formula.
Calculate the electrostatic force F by performing the multiplication and division as per the formula.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Coulomb's Law
Coulomb's Law describes the electrostatic force between two charged objects. It states that the force (F) is directly proportional to the product of the magnitudes of the charges (Q1 and Q2) and inversely proportional to the square of the distance (d) between them. The equation F = k(Q1Q2/d²) quantifies this relationship, where k is the electrostatic constant.
Recommended video:
Guided course
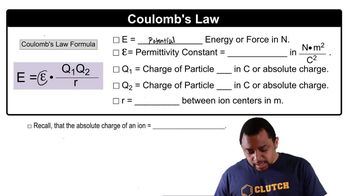
Coulomb's Law Concept 2
Units of Charge and Distance
In the context of electrostatics, charge is measured in Coulombs (C), while distance is typically measured in meters (m). The problem involves converting picometers (pm) to meters, as 1 pm equals 1×10⁻¹² m. Understanding these units is crucial for correctly applying Coulomb's Law and calculating the electrostatic force.
Recommended video:
Guided course
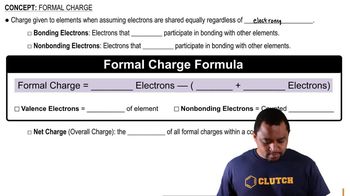
Formal Charge
Electrostatic Force Calculation
To calculate the electrostatic force between an electron and a proton, one must substitute the known values into Coulomb's Law. The charge of an electron is approximately -1.6×10⁻¹⁹ C, and that of a proton is +1.6×10⁻¹⁹ C. By using the distance of 1×10² pm (or 1×10⁻¹⁰ m), one can compute the force in Newtons, which indicates the strength of the attraction between these two fundamental particles.
Recommended video:
Guided course
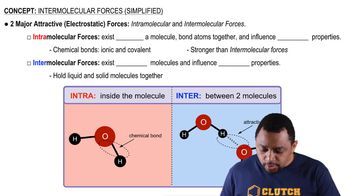
Intermolecular vs Intramolecular Forces
Related Practice
Textbook Question
Textbook Question
(b) What is the change in potential energy if the distance separating the two electrons is increased to 1.0 nm?
Textbook Question
(c) Does the potential energy of the two particles increase or decrease when the distance is increased to 1.0 nm?
Textbook Question
A sodium ion, Na+, with a charge of 1.6⨉10-19 C and a chloride ion, Cl - , with charge of -1.6⨉10-19 C, are separated by a distance of 0.50 nm. How much work would be required to increase the separation of the two ions to an infinite distance?
Textbook Question
A magnesium ion, Mg2+, with a charge of 3.2⨉10-19 C and an oxide ion, O2-, with a charge of -3.2⨉10-19 C, are separated by a distance of 0.35 nm. How much work would be required to increase the separation of the two ions to an infinite distance?
