State whether each of the following statements is true or false. Justify your answer in each case. (a) Electrolyte solutions conduct electricity because electrons are moving through the solution.
Ch.4 - Reactions in Aqueous Solution

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 4, Problem 15
We have learned in this chapter that many ionic solids dissolve in water as strong electrolytes; that is, as separated ions in solution. Which statement is most correct about this process? (a) Water is a strong acid and therefore is good at dissolving ionic solids. (b) Water is good at solvating ions because the hydrogen and oxygen atoms in water molecules bear partial charges. (c) The hydrogen and oxygen bonds of water are easily broken by ionic solids.
 Verified step by step guidance
Verified step by step guidance1
Identify the nature of water as a solvent. Water is a polar molecule, meaning it has a partial positive charge on the hydrogen atoms and a partial negative charge on the oxygen atom.
Consider the role of water's polarity in dissolving ionic solids. The partial charges on water molecules can interact with the positive and negative ions in an ionic solid.
Evaluate statement (a): Water is not a strong acid; it is a neutral solvent. Therefore, this statement is incorrect.
Evaluate statement (b): The partial charges on the hydrogen and oxygen atoms in water make it effective at solvating ions, as these charges can stabilize the ions in solution.
Evaluate statement (c): The bonds within water molecules are not easily broken by ionic solids. Instead, water's ability to dissolve ionic solids is due to its polarity and ability to solvate ions.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ionic Compounds and Solubility
Ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces. When these compounds dissolve in water, the ionic bonds are overcome by the interactions with water molecules, allowing the ions to separate and disperse in solution. This process is essential for understanding how ionic solids behave in aqueous environments.
Recommended video:
Guided course
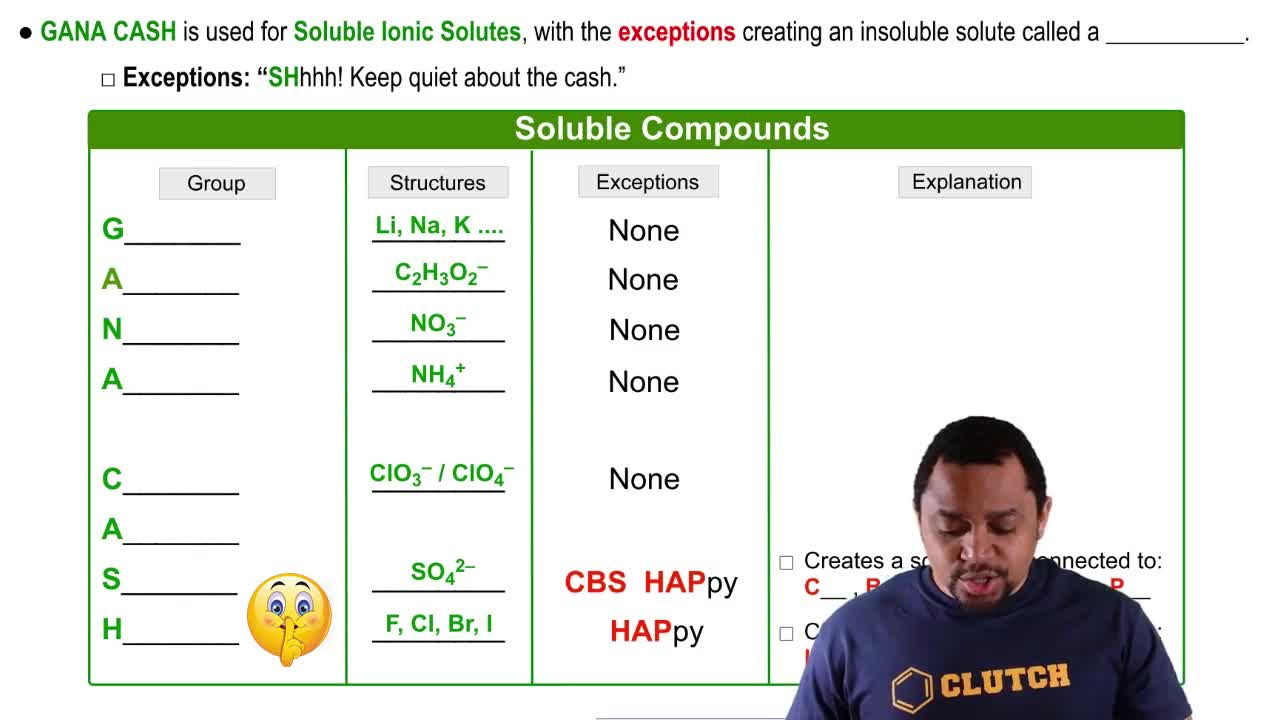
Soluble Ionic Solutes
Polarity of Water
Water is a polar molecule, meaning it has a partial positive charge on the hydrogen atoms and a partial negative charge on the oxygen atom. This polarity allows water molecules to interact effectively with charged ions, stabilizing them in solution through solvation. The ability of water to solvate ions is a key factor in its role as a solvent for ionic compounds.
Recommended video:
Guided course
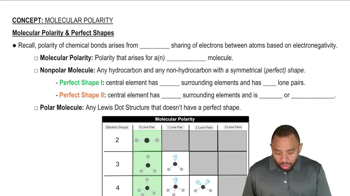
Molecular Polarity
Solvation Process
Solvation is the process by which solvent molecules surround and interact with solute ions or molecules. In the case of ionic solids dissolving in water, the water molecules surround the individual ions, effectively separating them from the solid lattice. This interaction is crucial for the dissolution of ionic compounds and is driven by the favorable energy changes associated with the formation of solute-solvent interactions.
Recommended video:
Guided course
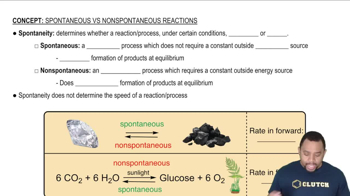
Spontaneity of Processes
Related Practice
Textbook Question
Textbook Question
State whether each of the following statements is true or false. Justify your answer in each case. (b) If you add a nonelectrolyte to an aqueous solution that already contains an electrolyte, the electrical conductivity will not change.
1
views
Textbook Question
Would you expect that an anion would be physically closer to the oxygen or to the hydrogens of water molecules that surround it in solution?
3
views
Textbook Question
Specify what ions are present in solution upon dissolving each of the following substances in water: a. FeCl2
Textbook Question
Specify what ions are present in solution upon dissolving each of the following substances in water: c. (NH4)2SO4
