We have learned in this chapter that many ionic solids dissolve in water as strong electrolytes; that is, as separated ions in solution. Which statement is most correct about this process? (a) Water is a strong acid and therefore is good at dissolving ionic solids. (b) Water is good at solvating ions because the hydrogen and oxygen atoms in water molecules bear partial charges. (c) The hydrogen and oxygen bonds of water are easily broken by ionic solids.
Ch.4 - Reactions in Aqueous Solution

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 4, Problem 17a
Specify what ions are present in solution upon dissolving each of the following substances in water: a. FeCl2
 Verified step by step guidance
Verified step by step guidance1
Identify the compound: FeCl2 is iron(II) chloride.
Determine the ions formed: When FeCl2 dissolves in water, it dissociates into its constituent ions.
Write the dissociation equation: FeCl2 (s) → Fe^{2+} (aq) + 2 Cl^{-} (aq).
Recognize the ions in solution: The ions present in the solution are Fe^{2+} and Cl^{-}.
Note the stoichiometry: For each formula unit of FeCl2, one Fe^{2+} ion and two Cl^{-} ions are produced.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Dissociation of Ionic Compounds
When ionic compounds like FeCl2 dissolve in water, they dissociate into their constituent ions. This process occurs because the polar water molecules surround and separate the individual ions, allowing them to disperse throughout the solution.
Recommended video:
Guided course

Ionic Compounds Naming
Ionic Composition of FeCl2
FeCl2 is composed of iron (Fe) and chloride (Cl) ions. Specifically, it contains one iron ion with a +2 charge (Fe²⁺) and two chloride ions, each with a -1 charge (Cl⁻). Understanding this composition is crucial for identifying the ions present in solution.
Recommended video:
Guided course
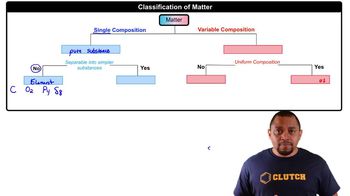
Matter Composition
Electrolytes in Solution
Electrolytes are substances that dissociate into ions in solution and can conduct electricity. The presence of Fe²⁺ and Cl⁻ ions from the dissociation of FeCl2 makes the solution an electrolyte, which is important for various chemical and biological processes.
Recommended video:
Guided course
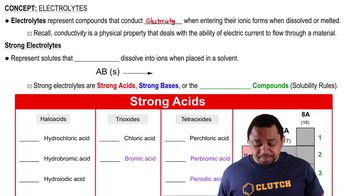
Electrolytes and Strong Acids
Related Practice
Textbook Question
1
views
Textbook Question
Would you expect that an anion would be physically closer to the oxygen or to the hydrogens of water molecules that surround it in solution?
3
views
Textbook Question
Specify what ions are present in solution upon dissolving each of the following substances in water: c. (NH4)2SO4
Textbook Question
Specify what ions are present upon dissolving each of the following substances in water: (a) MgI2
1
views
Textbook Question
Specify what ions are present upon dissolving each of the following substances in water: (b) K2CO3
