Textbook Question
Specify what ions are present in solution upon dissolving each of the following substances in water: a. FeCl2

 Verified step by step guidance
Verified step by step guidance

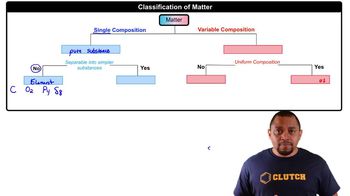

Specify what ions are present in solution upon dissolving each of the following substances in water: a. FeCl2
Specify what ions are present in solution upon dissolving each of the following substances in water: c. (NH4)2SO4
Specify what ions are present upon dissolving each of the following substances in water: (a) MgI2
Specify what ions are present upon dissolving each of the following substances in water: (c) HClO4
Specify what ions are present upon dissolving each of the following substances in water: (d) NaCH3COO
Formic acid, HCOOH, is a weak electrolyte. What solutes are present in an aqueous solution of this compound?