Give the name or condensed structural formula, as appropriate (e)
Ch.24 - The Chemistry of Life: Organic and Biological Chemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
All textbooks Brown 15th Edition
Brown 15th Edition Ch.24 - The Chemistry of Life: Organic and Biological Chemistry
Ch.24 - The Chemistry of Life: Organic and Biological Chemistry Problem 45c
Problem 45c
 Brown 15th Edition
Brown 15th Edition Ch.24 - The Chemistry of Life: Organic and Biological Chemistry
Ch.24 - The Chemistry of Life: Organic and Biological Chemistry Problem 45c
Problem 45cChapter 24, Problem 45c
Draw the condensed structure of the compounds formed by condensation reactions between
c. acetic acid and phenol.
Name the compound in each case.
 Verified step by step guidance
Verified step by step guidance1
Identify the functional groups involved in the condensation reaction: acetic acid contains a carboxylic acid group (-COOH) and phenol contains a hydroxyl group (-OH).
Understand that a condensation reaction between a carboxylic acid and an alcohol (or phenol) typically forms an ester and water.
Write the general reaction for esterification: R-COOH + R'-OH \rightarrow R-COOR' + H_2O.
Apply the specific reactants: acetic acid (CH_3COOH) and phenol (C_6H_5OH) to form the ester, which is phenyl acetate (CH_3COOC_6H_5).
Draw the condensed structural formula of phenyl acetate: CH_3COOC_6H_5, where the acetic acid part is CH_3COO- and the phenol part is -C_6H_5.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Condensation Reactions
Condensation reactions are chemical processes where two molecules combine to form a larger molecule, releasing a small molecule, often water. In organic chemistry, these reactions frequently involve functional groups such as alcohols and carboxylic acids, leading to the formation of esters or amides. Understanding this concept is crucial for predicting the products formed when acetic acid and phenol react.
Recommended video:
Guided course
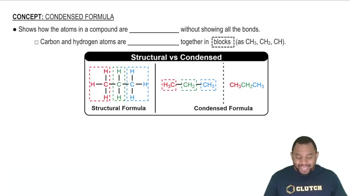
Condensed Formula
Functional Groups
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. In the case of acetic acid, the carboxylic acid group (-COOH) is key, while phenol contains a hydroxyl group (-OH). Recognizing these functional groups helps in understanding how they interact during condensation reactions to form new compounds.
Recommended video:
Guided course
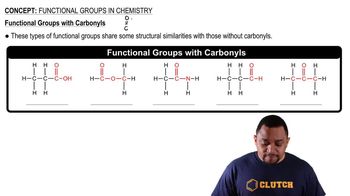
Carbonyl Functional Groups
Condensed Structural Formulas
Condensed structural formulas provide a simplified representation of a molecule, showing the arrangement of atoms and the connectivity between them without depicting all the bonds explicitly. This notation is particularly useful for illustrating the products of condensation reactions, as it allows for a clear and concise depiction of the resulting compounds formed from acetic acid and phenol.
Recommended video:
Guided course

Condensed Formula
Related Practice
Textbook Question
1
views
1
rank
Textbook Question
Describe the intermediate that is thought to form in the addition of a hydrogen halide to an alkene, using cyclohexene as the alkene in your description.
Textbook Question
Draw the condensed structure of the compounds formed by condensation reactions between
a. benzoic acid and ethanol
Name the compound in each case.
Textbook Question
Draw the condensed structure of the compounds formed by condensation reactions between
a. butanoic acid and methanol
Name the compound in each case.
Textbook Question
Draw the condensed structure of the compounds formed by condensation reactions between
b. benzoic acid and 2-propanol
Name the compound in each case.
Textbook Question
Write a balanced chemical equation using condensed structural formulas for the saponification (base hydrolysis) of
a. methyl propionate