Textbook Question
Give the name or condensed structural formula, as appropriate (e)
1
views
1
rank

 Brown 15th Edition
Brown 15th Edition Ch.24 - The Chemistry of Life: Organic and Biological Chemistry
Ch.24 - The Chemistry of Life: Organic and Biological Chemistry Problem 36
Problem 36 Verified step by step guidance
Verified step by step guidance
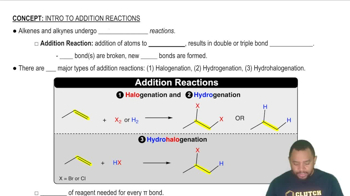


Give the name or condensed structural formula, as appropriate (e)
Draw the condensed structure of the compounds formed by condensation reactions between
a. benzoic acid and ethanol
Name the compound in each case.
Draw the condensed structure of the compounds formed by condensation reactions between
c. acetic acid and phenol.
Name the compound in each case.
Draw the condensed structure of the compounds formed by condensation reactions between
a. butanoic acid and methanol
Name the compound in each case.