Each of the following nuclei undergoes either beta decay or positron emission. Predict the type of emission for each: (a) tritium, 31H.
Ch.21 - Nuclear Chemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 21, Problem 22
One nuclide in each of these pairs is radioactive. Predict which is radioactive and which is stable: (a) 4020Ca and 4520Ca. Explain your choice in each case. (b) 12C and 14C. Explain your choice in each case. (c) lead-206 and thorium-230. Explain your choice in each case.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of nuclear stability. A nuclide's stability is influenced by the ratio of neutrons to protons (N/Z ratio) and the presence of magic numbers, which are numbers of nucleons that complete a shell within the nucleus.
Step 2: Analyze pair (a) 40_20Ca and 45_20Ca. Calculate the N/Z ratio for each nuclide. For 40_20Ca, the number of neutrons is 20, giving an N/Z ratio of 1. For 45_20Ca, the number of neutrons is 25, giving an N/Z ratio of 1.25. Compare these ratios to the ideal ratio for stability, which is close to 1 for lighter elements.
Step 3: Analyze pair (b) 12C and 14C. Calculate the N/Z ratio for each nuclide. For 12C, the number of neutrons is 6, giving an N/Z ratio of 1. For 14C, the number of neutrons is 8, giving an N/Z ratio of 1.33. Consider the stability of carbon isotopes and the fact that 14C is known to be radioactive.
Step 4: Analyze pair (c) lead-206 and thorium-230. Recognize that lead-206 is a stable isotope, often found as a product of radioactive decay chains, while thorium-230 is part of the decay series of uranium-238 and is radioactive.
Step 5: Conclude which nuclide in each pair is radioactive based on the N/Z ratio and known stability of isotopes. For pair (a), 45_20Ca is radioactive due to its higher N/Z ratio. For pair (b), 14C is radioactive as it is a known radioactive isotope. For pair (c), thorium-230 is radioactive as it is part of a decay series.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclear Stability
Nuclear stability refers to the ability of a nucleus to remain intact without undergoing radioactive decay. Stable nuclei have a balanced ratio of protons to neutrons, while unstable nuclei may have too many or too few neutrons, leading to decay. Understanding the stability of isotopes is crucial for predicting which nuclides are radioactive.
Recommended video:
Guided course
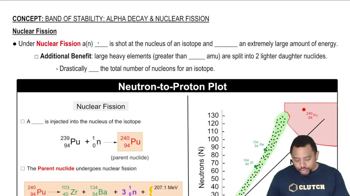
Band of Stability: Nuclear Fission
Isotopes
Isotopes are variants of a chemical element that have the same number of protons but different numbers of neutrons. This difference in neutron count can affect the stability of the nucleus. For example, carbon-12 is stable, while carbon-14 is radioactive due to its higher neutron count, which makes it prone to decay.
Recommended video:
Guided course

Isotopes
Decay Modes
Decay modes are the processes by which unstable nuclei release energy and particles to achieve stability. Common decay modes include alpha decay, beta decay, and gamma decay. Identifying the decay mode helps in understanding the nature of the radioactive nuclide and predicting its behavior over time.
Recommended video:
Guided course

Beta Decay
Related Practice
Textbook Question
1
views
Textbook Question
Each of the following nuclei undergoes either beta decay or positron emission. Predict the type of emission for each: b. 9938Sr
Textbook Question
Despite the similarities in the chemical reactivity of elements in the lanthanide series, their abundances in Earth's crust vary by two orders of magnitude. This graph shows the relative abundance as a function of atomic number. Which of the following statements best explains the sawtooth variation across the series? (a) The elements with an odd atomic number lie above the belt of stability. (b) The elements with an odd atomic number lie below the belt of stability. (c) The elements with an even atomic number have a magic number of protons. (d) Pairs of protons have a special stability.
2
views
